- A. V. Rozhkov, E. A. Katlenok, M. V. Zhmykhova, M. L. Kuznetsov, V. N. Khrustalev, K. I. Tugashov, N. A. Bokach, V. Yu. Kukushkin, Spodium bonding to anticrown-Hg3 boosts phosphorescence of cyclometalated-PtII complexes, Inorg. Chem. Front., 10 (2023); 493–510 doi: 10.1039/D2QI02047E.
- M. A. Kinzhalov, D. M. Ivanov, A. V. Shishkina, A. A. Melekhova, V. V. Suslonov, A. Frontera, V. Yu. Kukushkin, N. A. Bokach, Halogen bonding between metal-bound I3– and unbound I2: The trapped I2···I3– intermediate in the controlled assembly of copper(I)-based polyiodides, Inorg. Chem. Front., 10 (2023); doi: 10.1039/D2QI02634A.
- P.M. Tolstoy, E.Yu. Tupikina, “IR and NMR spectral diagnostics of hydrogen bond energy and geometry” in “Spectroscopy and Computation of Hydrogen-Bonded Systems”, Edited by Marek J. Wojcik, Y. Ozaki, 2023, John Wiley and Sons. pp. 345–407. DOI: 10.1002/9783527834914.ch12.
- K. Grzhegorzhevskii, M. Tonkushina, P. Gushchin, I. Gagarin, A. Ermoshin, K. Belova, A. Prokofyeva, A. Ostroushko, A. Novikov, Association of Keplerate-type polyoxometalate {Mo72Fe30} with tetracycline: nature of binding sites and antimicrobial action, Inorganics 11 (2023) 9; DOI: 10.3390/inorganics11010009.
- L. Savintseva, A. Avdoshin, S. Ignatov, A. Novikov, Theoretical study of charge mobility in crystal porphine and a computer design of a porphine-based semiconductive discotic liquid mesophase, Int. J. Mol. Sci. 24 (2023) 736; DOI: 10.3390/ijms24010736.
- A. Pulyalina, V. Rostovtseva, I. Faykov, N. Saprykina, A. Golikova, A. Fedorova, G. Polotskaya, A. Novikov, Impact of layered perovskite oxide La0.85Yb0.15AlO3 on structure and transport properties of polyetherimide, Int. J. Mol. Sci. 24 (2023) 715; DOI: 10.3390/ijms24010715.
- A. S. Novikov, Recent progress in the theoretical studies of the noncovalent interactions of supramolecular complexes with polyhalides and halometalates, Compounds 3 (2023) 27–36; DOI: 10.3390/compounds3010003.
- L. G. Lavrenova, A. I. Ivanova, L. A. Glinskaya, A. V. Artem’ev, A. N. Lavrov, A. S. Novikov, P. A. Abramov, Halogen bonding channels for magnetic exchange in Cu(II) complexes with 2,5-di(methylthio)-1,3,4-thiadiazole, Chem. Asian J. (2023) In press. DOI: 10.1002/asia.202201200.
- A. S. Smirnov, A. S. Mikherdov, R. M. Gomila, A. Frontera, V. Yu. Kukushkin, N. A. Bokach, Halogen bond-involving supramolecular assembly utilizing carbon as a nucleophilic partner of I···C non-covalent interaction, Chem. Asian J., 2023; doi: 10.1002/asia.202300037.
- A. S. Novikov, Non-covalent catalysts, Catalysts 13 (2023), 339; DOI: 10.3390/catal13020339.
- A. V. Artem’ev, E. P. Doronina, M. I. Rakhmanova, X. Hei, D. V. Stass, O. A. Tarasova, I. Yu. Bagryanskaya, D. G. Samsonenko, A. S. Novikov, N. A. Nedolya, J. Li, A family of CuI-based 1D polymers showing colorful short-lived TADF and phosphorescence induced by photo- and X-ray irradiation, Dalton Trans. (2023) In press. DOI: 10.1039/D3DT00035D.
- I. N. Klyukin, A. V. Kolbunova, A. S. Novikov, A. V. Nelyubin, A. P. Zhdanov, A. S. Kubasov, N. A. Selivanov, A. Y. Bykov, K. Y. Zhizhin, N. T. Kuznetsov, Synthesis of disubstituted carboxonium derivatives of closo-decaborate anion [2,6-B10H8O2CC6H5]−: theoretical and experimental study, Molecules 28 (2023) 1757; DOI: 10.3390/molecules28041757.
- E. S. Sheina, T. S. Shestakova, S. L. Deev, I. A. Khalymbadzha, P. A. Slepukhin, O. S. Eltsov, A. S. Novikov, V. A. Shevyrin, V. N. Charushin, O. N. Chupakhin, Mesomeric betaines based on adamantylated 1,2,4-triazolo[4,3-a]pyrimidin-5-ones: synthesis, structure and conversion into anionic N-heterocyclic carbenes, Chem. Asian J. (2023) In press. DOI: 10.1002/asia.202201306.
- A. S. Novikov, Recent progress in theoretical studies and computer modeling of non-covalent interactions, Crystals 13 (2023) 361; DOI: 10.3390/cryst13020361.
- N. S. Soldatova, V. V. Suslonov, D. M. Ivanov, M. S. Yusubov, G. Resnati, P. S. Postnikov, V. Yu. Kukushkin, Controlled halogen bond-involving assembly of double-σ-hole donating diaryliodonium cations and ditopic arene sulfonates, Crystal Growth & Design, 23 (2023) 413–423; doi: 10.1021/acs.cgd.2c01090.
- A. S. Novikov, Theoretical Studies and Computer Modeling of Supramolecular Chemical Systems: Structure, Properties and Reactivity. Chem. Proc. 12 (2022) 88; DOI: 10.3390/ecsoc-26-13717.
- K. V. Derkach, M. A. Gureev, A. A. Babushkina, V. N. Mikhaylov, I. O. Zakharova, A. A. Bakhtyukov, V. N. Sorokoumov, A. S. Novikov, M. Krasavin, A. O. Shpakov, I. A. Balova, Dual PTP1B/TC-PTP Inhibitors: Biological Evaluation of 3-(Hydroxymethyl)cinnoline-4(1H)-Ones, Int. J. Mol. Sci. 24 (2023) 4498; DOI: 10.3390/ijms24054498.
- A. S. Novikov, Non-Covalent Interactions in Polymers, Polymers 15 (2023) 1139; DOI: 10.3390/polym15051139.
- M. A. Bondarenko, D. A. Zherebtsov, A. S. Novikov, V. P. Fedin, S. A. Adonin, Two-dimensional Cu(II) 5-iodoisophthalate with a 1,2-bis(4-pyridyl)ethylene linker: crystal structure and features of electronic structure, J. Struct. Chem. 64 (2023) 112; DOI: 10.1134/S0022476623010079.
- I. I. Fedorova, N. S. Soldatova, D. M. Ivanov, K. A. Nikiforova, I. S. Aliyarova, M. S. Yusubov, P. M. Tolstoy, R. M. Gomila, A. Frontera, V. Yu. Kukushkin, P. S. Postnikov, G. Resnati, Benzothienoiodolium cations doubly bonded to anions via halogen-chalcogen and halogen-hydrogen supramolecular synthons, Crystal Growth & Design, 23 (2023); doi: 10.1021/acs.cgd.2c01485.
- A. V. Rozhkov, M. V. Zhmykhova, Y. V. Torubaev, E. A. Katlenok, V. Yu. Kukushkin, Bis(perfluoroaryl)chalcolanes ArF2Ch (Ch = S, Se, Te) as σιγμα/πι-hole donors for supramolecular applications based on noncovalent bonding, Crystal Growth & Design, 23 (2023); doi: 10.1021/acs.cgd.2c01454.
- A.A. Sysoeva, A.S. Novikov, M.V. Il'in, D.S. Bolotin, Solvent-modulated binding selectivity of reaction substrates to onium-based sigma-hole donors, Catal. Sci. Technol., (2023). DOI: 10.1039/D3CY00071K.
- S. A. Katkova, D. O. Kozina, K. S. Kisel, M. A. Sandzhieva, D. A. Tarvanen, S. V. Makarov, V. V. Porsev, S. P. Tunik, M. A. Kinzhalov, Cyclometalated platinum(ii) complexes with acyclic diaminocarbene ligands for OLED application, Dalton Trans., 2023. DOI: 10.1039/d3dt00080j.
- A. V. Semenov, S. V. Baykov, N. S. Soldatova, K. K. Geyl, D. M. Ivanov, A. Frontera, V. P. Boyarskiy, P. S. Postnikov, V. Yu. Kukushkin, Noncovalent chelation by halogen bonding in the design of metal-containing arrays: assembly of doubles-hole donating halolium with CuI-containing O,O-donors, Inorg. Chem. , 62 (2023); 10.1021/acs.inorgchem.3c00229.
- E. A. Katlenok, N. A. Semenov, M. L. Kuznetsov, N. A. Bokach, V. Yu. Kukushkin, A new look at the chalcogen bond: π-hole-based chalcogen (Se, Te) bonding which does not include a σ-hole interaction, Inorg. Chem. Front., 10 (2023); doi: 10.1039/D3QI00087G.
- I. A. Khodov, K. V. Belov, V. V. Sobornova, A. E. Batista de Carvalho Luís, A. A. Dyshin, P. M. Tolstoy, V. V. Mulloyarova, M. G. Kiselev, “Exploring the Conformational Equilibrium of Mefenamic Acid Released from Silica Aerogels via NMR Analysis”, Int. J. Mol. Sci. 24 (2023) 6882; DOI: 10.3390/ijms24086882.
- E. Yu. Tupikina, V. O. Korostelev, D. V. Krutin, P. M. Tolstoy, “Evolution of vibrational bands upon gradual protonation/deprotonation of arsinic acid H2AsOOH in media of different polarity”, Phys. Chem. Chem. Phys. 25 (2023) 8664-8675; DOI: 10.1039/D2CP06060D.
- U. M. Leksina, A. Y. Shishov, V. V. Mulloyarova, A. M. Puzyk, P. M. Tolstoy, M. Vokuev, E. D. Glushkov, V. G. Petrov, P. I. Matveev, “A new deep eutectic solvent based on diphenylguanidine for the effective extraction of pertechnetate anion”, Sep. Purif. Technol. (2023), available online; DOI: 10.1016/j.seppur.2023.123824.
- Y. S. Bortnevskaya, N. A. Shiryaev, N. S. Zakharov, O. O. Kitoroage, M. A. Gradova, N. Y. Karpechenko, A. S. Novikov, E. D. Nikolskaya, M. R. Mollaeva, N. G. Yabbarov, N. A. Bragina, K. A. Zhdanova, Synthesis and Biological Properties of EGFR-Targeted Photosensitizer Based on Cationic Porphyrin, Pharmaceutics 15 (2023) 1284; DOI:10.3390/pharmaceutics15041284.
- I. F. Sakhapov, A. A. Zagidullin, A. B. Dobrynin, I. A. Litvinov, D. G. Yakhvarov, M. A. Bondarenko, A. S. Novikov, V. P. Fedin, S. A. Adonin, Crystal Structures of 3,3′,5,5′-Tetrabromo-4,4′-bipyridine and Co(II) Coordination Polymer Based Thereon, Crystals 13 (2023) 704; DOI: 10.3390/cryst13040704.
- A. A. Eliseeva, M. A. Khazanova, A. M. Cheranyova, I. A. Aliyarova, R. I. Kravchuk, E. S. Oganesyan, A. V. Ryabykh, O. A. Maslova, D. M. Ivanov, S. A. Beznosyuk, Metal-involving halogen bonding confirmed using DFT calculations with periodic boundary conditions, Crystals, 13 (2023), 712; DOI: 10.3390/cryst13050712.
- E. V. Podrezova, A. A. Okhina, A. D. Rogachev, S. V. Baykov, A. Kirschning, M. S. Yusubov, N. S. Soldatova, P. S. Postnikov, Ligand-free Ullmann-type arylation of oxazolidinones by diaryliodonium salts. Org. Biomol. Chem., 21 (2023), 1952–1957; DOI: 10.1039/D2OB02122F.
- E. O. Sinegubova, O. A. Kraevaya, A. S. Volobueva, A. V. Zhilenkov, A. F. Shestakov, S. V. Baykov, S. V. P. A. Troshin, V. V. Zarubaev, Water-Soluble Fullerene C60 Derivatives Are Effective Inhibitors of Influenza Virus Replication. Microorganisms, 11 (2023), 681; DOI: 10.3390/microorganisms11030681.
- S. V. Baykov, A. A. Shetnev, A. V. Semenov, S. O. Baykova, V. P. Boyarskiy, Room Temperature Synthesis of Bioactive 1,2,4-Oxadiazoles. Int. J. Mol. Sci., 24 (2023), 5406; DOI: 10.3390/ijms24065406.
- A. Shetnev, A. Kotov, A. Kunichkina, I. Proskurina, S. Baykov, M. Korsakov, A. Petzer, J. P. Petzer, Monoamine Oxidase Inhibition Properties of 2,1-Benzisoxazole Derivatives. Mol. Divers. (2023); DOI: 10.1007/s11030-023-10628-4.
- K. V. Deriabin, E. A. Golovenko, N. Antonov, S. Baykov, V. Boyarskiy, R. M. Islamova, Platinum-Macrocatalyst for Heterogeneous Si–O Dehydrocoupling. Dalt. Trans. (2023), in press; DOI: 10.1039/D3DT00651D.
- Baykova, S. O.; Geyl, K. K.; Baykov, S. V.; Boyarskiy, V. P. Synthesis of 3-(Pyridin-2-Yl)Quinazolin-2,4(1H,3H)-Diones via Annulation of Anthranilic Esters with N-Pyridyl Ureas. Int. J. Mol. Sci., 24 (2023), 7633; DOI: 10.3390/ijms24087633.
- I.N. Klyukin, A.V. Kolbunova, A.S. Novikov, A.P. Zhdanov, K.Y. Zhizhin, N.T. Kuznetsov, Theoretical Insight on the Formation Mechanism of a Trisubstituted Derivative of Closo-Decaborate Anion [B10H7O2CCH3(NCCH3)]0, Inorganics 11 (2023) 201; DOI: 10.3390/inorganics11050201.
- I.S. Fomenko, O.S. Koshcheeva, N.I. Kuznetsova, T.V. Larina, M.I. Gongola, M. Afewerki, P.A. Abramov, A.S. Novikov, A.L. Gushchin, Novel Copper(II) Complexes with BIAN Ligands: Synthesis, Structure and Catalytic Properties of the Oxidation of Isopropylbenzene, Catalysts 13 (2023) 849; DOI: 10.3390/catal13050849.
- D.S. Suslov, M.V. Bykov, M.V. Pakhomova, T.S. Orlov, Z.D. Abramov, A.V. Suchkova, I.A. Ushakov, P.A. Abramov, A.S. Novikov, Novel Route to Cationic Palladium(II)–Cyclopentadienyl Complexes Containing Phosphine Ligands and Their Catalytic Activities, Molecules 28 (2023) 4141; DOI: 10.3390/molecules28104141.
- E.S. Starnovskaya, M.I. Savchuk, R. Aluru, D.S. Kopchuk, A.F. Khasanov, O.S. Taniya, A.S. Novikov, A.A. Kalinichev, S. Santra, G.V. Zyryanov, B.C. Ranu, Carbazole/fluorene substituted 5-phenyl-2,2'-bipyridine D-π-A fluorophores: Photophysical data, hyperpolarizability and CT-indices, New J. Chem. (2023) In press.; DOI: 10.1039/D3NJ00394A.
- M.R. Guda, M.I. Valieva, D.S. Kopchuk, R. Aluru, A.F. Khasanov, O.S. Taniya, A.S. Novikov, G.V. Zyryanov, B. C. Ranu, One-pot synthesis and photophysical studies of Α-cycloamino-substituted 5-aryl-2,2'-bipyridines, J. Fluoresc. (2023) In press.; DOI: 10.1007/s10895-023-03304-1.
- M.S. Mohammed, I.S. Kovalev, N.V. Slovesnova, L.K. Sadieva, V.A.Platonov, A.S. Novikov, S. Santra, J.E. Morozova, G.V. Zyryanov, V.N. Charushin, B.C. Ranu, Polyaromatic hydrocarbon (PAH)-based aza-POPOPs: synthesis, photophysical studies, and nitroanalyte sensing abilities, Int. J. Mol. Sci. 24 (2023) 10084; DOI: 10.3390/ijms241210084.
- R.A. Popov, A.S. Novikov, V.V. Suslonov, V.P. Boyarskiy, Molecular switching through chalcogen-bond-induced isomerization of binuclear (diaminocarbene)PdII complexes, Inorganics 11 (2023) 255; DOI: 10.3390/inorganics11060255.
- I.A. Shentseva, N.A. Korobeynikov, A.N. Usoltsev, I.D. Gorokh, A.S. Novikov, I.V. Korolkov, M.N. Sokolov, S.A. Adonin, Haloantimonate(III) complexes with halogen-substituted quinolinium cations: structural features of non-covalent interactions in solid state, Polyhedron (2023) In press.; DOI: 10.1016/j.poly.2023.116505.
- N.F. Romashev, I.V. Bakaev, V.I. Komlyagina, P.A. Abramov, I.V. Mirzaeva, V.A. Nadolinny, A.N. Lavrov, N.B. Kompan’kov, A.A. Mikhailov, I.S. Fomenko, A.S. Novikov, M.N. Sokolov, A.L. Gushchin, Iridium complexes with BIAN-type ligands: synthesis, structure and redox chemistry, Int. J. Mol. Sci. 24 (2023) 10457; DOI: 10.3390/ijms241310457.
- M. V. Kashina, M. A. Kinzhalov, E. Yu. Tupikina, V. Yu. Kukushkin, Bent and linear noncovalent M···S=C=N–M’ interactions. The case of palladium(II) and platinum(II) thiocyanate species, Crystal Growth & Design, 23 (2023); doi:10.1021/acs.cgd.3c00116.
- E. A. Katlenok, M. L. Kuznetsov, A. V. Cherkasov, D. M. Kryukov, N. A. Bokach, V. Yu. Kukushkin, Metal-involved C···dz2-PtII tetrel bonding as a principal component of the stacking interaction between arenes and the platinum(II) square-plane, Inorg. Chem. Front., 10 (2023); doi: 10.1039/D3QI00555K.
- Yu. N. Toikka, G. L. Starova, V. V. Suslonov, R. M. Gomila, A. Frontera, V. Yu. Kukushkin, N. A. Bokach, Combined sigma- and pi-hole donor properties of perfluorinated iodo(or bromo)benzenes: halogen bonding and pi-hole interactions in cocrystals including Cu4I4 clusters, Crystal Growth & Design, 23 (2023); doi: 10.1021/acs.cgd.3c00412.
- N. V. Shcherbakov, P. F. Kotikova, D. V. Dar’in, V. Yu. Kukushkin, A. Yu. Dubovtsev, Gold-catalyzed annulation of ynamides with aminocarbonyls as a route to 2-aminoquinolines diversely substituted at the 4th-position, Adv. Synth. Catal., 365 (2023); doi: 10.1002/adsc.202300484.
- A.A. Peshkov, D. Gapanenok, A. Puzyk, N. Amire, A.S. Novikov, S.D. Martynova, S. Kalinin, D. Dar’in, V.A. Peshkov, M. Krasavin, Substrate-Controlled Three-Component Synthesis of Diverse Fused Heterocycles, J. Org. Chem. (2023) In press. DOI: 10.1021/acs.joc.3c00497.
- M.S. Mohammed, I.S. Kovalev, N.V. Slovesnova, L.K. Sadieva, V.A. Platonov, G.A. Kim, R. Aluru, A.S. Novikov, O.S. Taniya, V.N. Charushin, (1-(4-(5-Phenyl-1,3,4-oxadiazol-2-yl)phenyl)-1H-1,2,3-triazol-4-yl)-methylenyls α,ω-Bisfunctionalized 3- and 4-PEG: Synthesis and Photophysical Studies, Molecules 28 (2023) 5256; DOI: 10.3390/molecules28135256.
- E.A. Lystsova, A.S. Novikov, M.V. Dmitriev, A.N. Maslivets, E.E. Khramtsova, Approach to Pyrido[2,1-b][1,3]benzothiazol-1-ones via In Situ Generation of Acyl(1,3-benzothiazol-2-yl)ketenes by Thermolysis of Pyrrolo[2,1-c][1,4]benzothiazine-1,2,4-triones, Molecules 28 (2023) 5495; DOI: 10.3390/molecules28145495.
- A. S. Smirnov, M. A. Kinzhalov, R. M. Gomila, A. Frontera, N. A. Bokach, V. Yu. Kukushkin, Isocyanide π-hole interactions supported by aurophilic forces, Crystals, 13 (2023) 1177; doi: 10.3390/cryst13081177.
- A. D. Radzhabov, N. S. Soldatova, D. M. Ivanov, M. S. Yusubov, V. Yu. Kukushkin, P. S. Postnikov, Metal-free and atom-efficient protocol for diarylation of selenocyanate by diaryliodonium salts, Org. Biomol. Chem., 21 (2023); doi: 10.1039/D3OB00833A.
- N. A. Zhestkij, A. S. Efimova, Y. Kenzhebayeva, M. V. Dmitriev, A. S. Novikov, I. D. Yushina, A. Krylov, M. V. Timofeeva, A. N. Kulakova, N. V. Glebova, A. A. Krasilin, S. A. Shipilovskih, V. A. Milichko, Nonlinear Metal–Organic Framework Crystals for Efficient Multicolor Coherent Optical Emission, Adv. Optical Mater. (2023) In press. DOI: 10.1002/adom.202300881.
- A. S. Novikov, Symmetry in Quantum and Computational Chemistry: Volume 2, Symmetry 15 (2023) 1472; DOI: 10.3390/sym15081472.
- E. E. Bardina, E. V. Makotchenko, K. P. Birin, I. A. Baidina, T. S. Sukhikh, A. S. Novikov, Y. G. Gorbunova, A. Yu. Tsivadze, A. L. Gushchin, Noncovalent interactions in gold(III) tetrakis(4-butoxyphenyl)porphyrinate structures, CrystEngComm (2023) In press. DOI: 10.1039/D3CE00428G.
- M. Shahab, G. Zheng, A. Khan, D. Wei, A. S. Novikov, Machine Learning-Based Virtual Screening and Molecular Simulation Approaches Identified Novel Potential Inhibitors for Cancer Therapy, Biomedicines 11 (2023) 2251; DOI: 10.3390/biomedicines11082251.
- V. G. Nenajdenko, A. A. Kazakova, A. S. Novikov, N. G. Shikhaliyev, A. M. Maharramov, A. M. Qajar, G. T. Atakishiyeva, A. A. Niyazova, V. N. Khrustalev, A. V. Shastin, A. G. Tskhovrebov, Copper-Catalyzed Reaction of N-Monosubstituted Hydrazones with CBr4: Unexpected Fragmentation and Mechanistic Study, Catalysts 13 (2023) 1194; DOI: 10.3390/catal13081194.
- A. Dogaru, A. A. Apostol, C. Maxim, M. Raduca, A. S. Novikov, A. Nicolescu, C. Deleanu, S. Nica, M. Andruh, Halogen bonded supramolecular assemblies constructed from azulene derivatives and perfluorinated di-/triiodobenzenes, CrystEngComm (2023) In press. DOI: 10.1039/D3CE00752A.
- A. S. Smirnov, E. A. Katlenok, A. S. Mikherdov, M. A. Kryukova, N. A. Bokach, V. Yu. Kukushkin, Halogen bonding involving isomeric isocyanide/nitrile groups, Int. J. Mol. Sci., 24 (2023) 13324; doi: 10.3390/ijms241713324.
- N. A. Korobeynikov, A. N. Usoltsev, M. N. Sokolov, A. S. Novikov, T. S. Sukhikh, S. A. Adonin, Halogen bonding in chloroiodates(III), CrystEngComm (2023) In press. DOI: 10.1039/D3CE00400G.
- A. S. Novikov, Computer Modeling and Machine Learning in Chemistry and Materials Science: From Properties and Reactions of Small Organic and Inorganic Molecules to the Smart Design of Polymers and Composites, Compounds 3 (2023) 459; DOI: 10.3390/compounds3030034.
- Agafonova A. V., Golubev A. A., Smetanin I. A., Khlebnikov A. F., Spiridonova D. V., Novikov M. S. Divergent Synthesis of Pyrazolo[1,5-a]pyridines and Imidazo[1,5-a]pyridines via Reagent-Controlled Cleavage of the C–N or C–C Azirine Bond in 2-Pyridylazirines. Org. Lett. 2023, DOI: 10.1021/acs.orglett.3c02696.
- Mammeri O. A., Baranov I. M., Ivanov A. Yu., Boyarskaya I. A., Vasilyev A. V. Synthesis of 2-(5H)-furanones by cyclization of alkyl allene carboxylates in triflic acid. Tetrahedron 2023, 146, 133649. DOI: 10.1016/j.tet.2023.133649
- Il’in, M.V., Polonnikov, D.A., Novikov, A.S., Sysoeva, A.A., Safinskaya, Y.V., Bolotin, D.S. Influence of Coordination to Silver(I) Centers on the Activity of Heterocyclic Iodonium Salts Serving as Halogen-Bond-Donating Catalysts. ChemPlusChem 2023, e202300304. DOI: 10.1002/cplu.202300304.
- Pankova, A.S., Golubev, P., Molin, I.A., Rostovskii, N.V. Regioselective Synthesis of 2-Trimethylsilyl-4H-pyran-4-ones from 1-Ethoxy(hydroxy)-5-(trimethylsilyl)pentenynones. Eur. J. Org. Chem. 2023, 26, e202300573. DOI: 10.1002/ejoc.202300573.
- Vidyakina, A.A., Shtyrov, A.A., Ryazantsev, M.N., Khlebnikov, A. F., Kolesnikov, I. E., Sharoyko, V. V., Spiridonova, D. V., Balova, I. A., Bräse, S., Danilkina, N.A. Development of Fluorescent Isocoumarin-Fused Oxacyclononyne – 1,2,3-Triazole Pairs. Chem. Eur. J. 2023, 29, e202300540. DOI: 10.1002/chem.202300540.
- Galenko, E.E., Novikov, M.S., Khlebnikov, A.F. [2+2] Cycloaddition/Retro-Electrocyclization/Decarboxylation Reaction Sequence: Access to 4-Aminopyridines from Methylideneisoxazolones and Ynamines. J. Org. Chem. 2023, 88, 8854–8864. DOI: 10.1021/acs.joc.3c00654.
- Silaichev, P.S., Beryozkina, T.V., Ilkin, V., Novikov, M.S., Dehaen, W., Bakulev, V.A. Tandem Cycloaddition of Azides to 3,3-Diaminoacrylonitriles (2-Cyanoacetamidines) and Cornforth-Type Rearrangement as an Approach to 5-Amino-1,2,3-triazole-4-N-substituted Imidamides. J. Org. Chem. 2023, 88, 8163–8174. DOI: 10.1021/acs.joc.3c00151.
- Vasilchenko, D.S., Agafonova, A.V., Simdianov, I.V., Koronatov, A. N., Sakharov, P.A., Romanenko, I. A., Rostovskii, N.V., Khlebnikov, A.F., Novikov, M.S. 2H-1,2,3-triazole-4-carboxylic acids via Ru(II)-catalyzed rearrangement of 4-hydrazonoisoxazol-5-ones. Tetrahedron Lett. 2023, 123, 154580. DOI: 10.1016/j.tetlet.2023.154580.
- Zakharov, T.N., Sakharov, P.A., Novikov, M.S., Khlebnikov, A.F., Rostovskii, N.V. Triethylamine-Promoted Oxidative Cyclodimerization of 2H-Azirine-2-carboxylates to Pyrimidine-4,6-dicarboxylates: Experimental and DFT Study. Molecules 2023, 28, 4315. DOI: 10.3390/molecules28114315.
- Taishev, A.E., Galenko, E.E., Novikov, M.S., Khlebnikov, A.F. Simple Access to Isoxazole-Containing Heterocyclic Hybrids: Isoxazole/Oxazole and Isoxazole/Pyridine. Russ. J. Gen. Chem. 2023, 93, 1246–1260. DOI: 10.1134/s1070363223050250.
- Titov, G.D., Antonychev, G.I., Novikov, M.S., Khlebnikov, A.F., Rogacheva, E. V., Kraeva, L.A., Rostovskii, N.V. Gold vs Light: Chemodivergent Reactivity of Diazoesters toward 2H-Azirine-2-carboxylic Acids. Org. Lett. 2023, 25, 2707–2712. DOI: 10.1021/acs.orglett.3c00823.
- Galenko, E.E., Zanakhov, T.O., Novikov, M.S., Khlebnikov, A.F. Metal carbonyl mediated rearrangement of 5-(2-oxoalkyl)-1,2,4-oxadiazoles: synthesis of fully substituted pyrimidines. Org. Biomol. Chem. 2023, 21, 2990–3001. DOI: 10.1039/D3OB00148B.
- Galenko, E.E., Zanakhov, T.O., Novikov, M.S., Khlebnikov, A.F. Pd-catalyzed heteroannulation of isoxazoles: Convergent synthesis of isoxazolo[5,4-c]quinolones. Tetrahedron Lett. 2023, 114, 154270. DOI: 10.1016/j.tetlet.2022.154270.
- Agafonova, A.V., Novikov, M.S., Khlebnikov, A.F. 5-Chloroisoxazoles: A Versatile Starting Material for the Preparation of Amides, Anhydrides, Esters, and Thioesters of 2H-Azirine-2-carboxylic Acids. Molecules 2023, 28, 275. DOI: 10.3390/molecules28010275.
- Govdi, A.I., Anisimov, S.O., Danilkina, N.A., Bunev, A.S., Balova, I.A. Acyclic enediynes fused to triazole and benzothiophene containing propargylamine moieties. Mendeleev Commun. 2023, 33, 328–330. DOI: 10.1016/j.mencom.2023.04.010.
- Babushkina, A.A., Mikhailov, V.N., Ogurtsova, A.D., Bunev, A. S., Sorokoumov, V.N., Balova, I.A. The Richter reaction in the synthesis of combretastatin analogs. Russ. Chem. Bull. 2023, 72, 1012–1022. DOI: 10.1007/s11172-023-3866-3.
- Derkach, K.V., Gureev, M.A., Babushkina, A.A., Mikhaylov, V.N., Zakharova, I.O., Bakhtyukov, A.A., Sorokoumov, V.N., Novikov, A.S., Krasavin, M., Shpakov, A.O., Balova, I.A. Dual PTP1B/TC-PTP Inhibitors: Biological Evaluation of 3-(Hydroxymethyl)cinnoline-4(1H)-Ones. Int. J. Mol. Sci. 2023, 24, 4498. DOI: 10.3390/ijms24054498.
- Efremova, M.M., Rumyantsev, A.M., Babitova, E.S., Ianshina, T.M., Govdi, A.I. Synthesis of 5-ethynylisoxazoles based on 1,3-dipolar cycloaddition reactions of nitrile oxides with conjugated diynes. Russ. Chem. Bull. 2023, 72, 1717–1721. DOI: 10.1007/s11172-023-3952-5.
- Cells Ianshina, T., Sidorin, A., Petrova, K., Shubert, M., Makeeva, A., Sambuk, E., Govdi, A., Rumyantsev, A., Padkina, M. Effect of Methionine on Gene Expression in Komagataella phaffii. Microorganisms 2023, 11, 877. DOI: 10.3390/microorganisms11040877.
- Sokolov, A.A., Egorov, D.M., Pronina, Y.A., Ramsh, S.M., Stepakov, A.V. 1,3-Dipolar Cycloaddition of Stable Azomethine Ylide Based on Ninhydrin and L-Proline to Phosphorylated Acetylenes. Russ. J. Gen. Chem. 2023, 93, 1694–1699. DOI: 10.1134/S1070363223070083
- Pevzner, L.M., Makhneva, E.A., Shmakov, S.V., Petrov, M.L., Stepakov, A.V., Boitsov, V.M. In Vitro Activity of Organochalcogen Compounds: IV. Synthesis and Cytotoxic Effect of 4-(1,2,3-Thiadiazol-4-yl)furans Against HeLa, Sk-mel-2, and B16 Tumor Cell Lines. Russ. J. Gen. Chem. 2023, 93, 1513–1522. DOI: 10.1134/S1070363223060245.
- Pevzner, L.M., Ostrovskaya, A.A., Petrov, M.L., Stepakov, A.V. Synthesis of Ethyl 2-Methyl-4-(diethoxyphosphoryl)-4,7-dihydro-5H-thiopyrano[3,4-b]furan-5-carboxylate and Its Functionalization at the Position 3. Russ. J. Gen. Chem. 2023, 93, 827–840. DOI: 10.1134/S1070363223040084.
- Pronina, Y.A., Stepakov, A.V., Popova, E.A., Boitsov, V.M., Baichurin, R.I., Selivanov, S.I. Diastereomers of Cyclopropa[a]pyrrolizine Spiro-fused with a Benzo[4,5]imidazo[1,2-a]indole Fragment: Structure Determinations Using NMR Methods. Appl. Magn. Reson. 2023 DOI: 10.1007/s00723-023-01608-w.
- Komolova, D.D., Pronina, Y.A., Lozovskiy, S.V., Selivanov, S.I., Ponyaev, A.I., Filatov, A.S., Boitsov, V.M., Stepakov, A.V. Palladium-Catalyzed Oxidative Cycloaddition of Quinazoline-2,4(1H,3H)-diones and Diarylalkynes via C-H/N-H Activation. Synthesis 2023 DOI: 10.1055/a-2105-2850.
- Makeeva, D.V., Antipova, K.S., Solovyeva, E.V., Morgacheva, V.P., Kolobova, E.A., Kartsova, L.A. Multilayer Coatings Based On Citrate-Stabilized Gold Nanoparticles and Polydiallyldimethylammonium Chloride for the Electrophoretic Separation of Carboxylic Acids. J. Anal. Chem. 2023, 78, 361–371. DOI: 10.1134/S1061934823030085.
- Kartsova, L.A., Maliushevska, A.V., Kolobova, E.A. Analytical Capabilities of the Determination of Carbohydrates by Chromatographic and Electrophoretic Methods. J. Anal. Chem. 2023, 78, 144–161. DOI: 10.1134/S1061934823020041.
- Karpitskiy, D.A., Bessonova, E.A., Shishov, A.Y., Kartsova, L.A. Selective extraction of plant bioactive compounds with deep eutectic solvents: Iris sibirica L. as example. Phytochem. Anal. 2023 DOI: 10.1002/pca.3272.
- Kravchenko, A.V., Kolobova, E.A., Kechin, A.A., Kartsova, L.A. Development of a capillary electrophoretic method for determination of ketorolac enantiomers in human plasma using cationic β-cyclodextrin derivative as a chiral selector. J. Sep. Sci. 2023, 46, 2200601. DOI: 10.1002/jssc.202200601.
- Zakusilo, D.N., Evstigneyev, E.I., Ivanov, A.Y., Mazur, A.S., Bessonova, E.A., Mammeri, O.A., Vasilyev, A.V. Structure of oxidized hydrolysis lignin. J. Wood Chem. Technol. 2023, 43, 103–115. DOI: 10.1080/02773813.2023.2187064.
- Zenkevich, I.G. Linear Correlations of the Gas Chromatographic Retention Indices of Compounds from Various Taxonomic Groups. J. Anal. Chem. 2023, 78, 766–775. DOI: 10.1134/S1061934823040160.
- Zenkevich, I.G., Deruish, A., Nikitina, D.A. Features of the Dependence of the Retention Indices of Sorbates in Reversed-Phase High-Performance Liquid Chromatography on the Content of Organic Solvents in the Eluent. Russ. J. Phys. Chem. A 2023, 97, 1007–1017. DOI: 10.1134/S0036024423050321.
- Deruish, A., Karakashev, G.V., Ukolov, A.I., Zenkevich, I.G. Hydrolytic Stability of Unsubstituted Hydrazones of Aromatic Carbonyl Compounds in Reversed-Phase HPLC. J. Anal. Chem. 2023, 78, 222–230. DOI: 10.1134/S106193482302003X.
- Savina, I.A., Gusev, D.M., Zimina, T.V., Golovanov, A.A., Zenkevich, I.G. Comparison of Gas-Chromatographic Retention Parameters of Aliphatic Enyne Alcohols with the Data for Their Structural Analogues. J. Anal. Chem. 2023, 78, 74–81. DOI: 10.1134/s1061934822120139.
- Zenkevich, I.G., Baranov, D.A. Gas-Chromatographic Identification of Unusual Unstable Products of the Partial Hydrolysis of Tetraethoxysilane. J. Anal. Chem. 2023, 78, 82–90. DOI: 10.1134/S1061934823010148.
- Zenkevich, I.G., Derouiche, A., Nikitina, D.A. Evidence for the Hydration of Some Organic Compounds during Reverse-Phase HPLC Analysis. Molecules 2023, 28, 734. DOI: 10.3390/molecules28020734.
- Ignatova, I.I., Khoroshilova, O.V., Vasilyev, A.V. Aluminium trichloride-promoted tandem hydroarylation–ionic hydrogenation of 3-arylpropynoic acid derivatives and 4-phenylbut-3-yn-2-one. Mendeleev Commun. 2023, 33, 27–29. DOI: 10.1016/j.mencom.2023.01.008.
- Samandarsangari, M., Kozina, D.O., Sokolov, V.V., Komarova, A.D., Shirmanova, M.V., Kritchenkov, I.S., Tunik, S.P. Biocompatible Phosphorescent O2 Sensors Based on Ir(III) Complexes for In Vivo Hypoxia Imaging. Biosensors 2023, 13, 680. DOI: 10.3390/bios13070680.
- Novichikhina, N.P., Pantykina, D.A., Shestakov, A.S., Potapov, A.Yu., Ledenyova, I.V., Kuznetsov, M.A., Shikhaliev, K.S. Allylic and Retro-Allylic Rearrangements upon Bromination of 8,9-Substituted 4,4,6-Trimethyl-4H-Pyrrolo[3,2,1-ij]Quinoline-1,2-Diones. New Aspects and Synthetic Applications. ChemistrySelect 2023, 8, e202203981. DOI: 10.1002/slct.202203981.
- Solovyeva, E.V., Odintsova, O.V., Svinko, V.O., Makeeva, D.V., Danilov, D.V. Hydroxyapatite-nanosilver composites with plasmonic properties for application in surface-enhanced Raman spectroscopy. Mater. Today Commun. 2023, 35, 105908. DOI: 10.1016/j.mtcomm.2023.105908.
- P. Debnath, P. Debnath, S. Roy, M. B. Devi, M. M. Devi, K. Sarangthem, S. S. Singh, M. Roy, A. S. Novikov, T. K. Misra, Azo-benzoic acid derivatives directed dinuclear and tetranuclear association of trimethyltin(IV) complex components and their biological activities, Inorg. Chim. Acta 559 (2024) 121805; DOI: 10.1016/j.ica.2023.121805.
- W.K.A. Al-Ithawi, R. Aluru, A.V. Baklykov, A.F. Khasanov, I.S. Kovalev, I.L. Nikonov, D.S. Kopchuk, A.S. Novikov, S. Santra, G.V. Zyryanov, B.C. Ranu, Mechanosynthesis of Polyureas and Studies of Their Responses to Anions, Polymers 15 (2023) 4160; DOI: 10.3390/polym15204160.
- K.A. Zhdanova, A.A. Zaytsev, M.A. Gradova, O.V. Gradov, A.V. Lobanov, A.S. Novikov, N.A. Bragina, Synthesis, Photophysical Properties, and Toxicity of o-Xylene-Bridged Porphyrin Dimers, Inorganics 11 (2023) 415; DOI: 10.3390/inorganics11100415.
- V. A. Rozentsvet, D. M. Ulyanova, N. A. Sablina, R. V. Brunilin, P. M. Tolstoy, Synthesis of Low Molecular Weight Butadiene Polymers Using Cationic Catalytic Systems Based on Diethylaluminum Chloride, Kinetics and Catalysis 64 (2023) 55; DOI: 10.1134/S0023158423010068.
- K. Sotiriadis, A. Mazur, P. Tolstoy, R. Ševčík, Exposure of Portland-Limestone Cement – Metakaolin Paste to Cold Chloride-Sulfate Environment: NMR Spectroscopy Assessment of Structural Changes in Hydrated Phases and Relation to Chloride Ingress, RILEM Bookseries 44 (2023) 966; DOI: 10.1007/978-3-031-33187-9_88.
- V. А. Rozentsvet, D. M. Ulyanova, N. А. Sablina, P. M. Tolstoy, M. G. Kuznetsov, Cationic polymerization of butadiene with isomerization of the initiator structure, Russ. Chem. Bull. 72 (2023) 2180; DOI: 10.1007/s11172-023-4014-8.
- V. A. Rozentsvet, D. M. Ulyanova, N.A. Sablina, S. V. Kostjuk, N. V. Sidorenko, P. M. Tolstoy, Diethylaluminum chloride-co-initiated cationic polymerization of isoprene: dramatic effect of the nature of alkyl halide on the properties of synthesized polymers, J. Macromol. Sci. A 60 (2023) 705; DOI: 10.1080/10601325.2023.2257739.
- K. Sotiriadis, A. Mazur, P. M. Tolstoy, D. Frankeova, Chloride effect on sulfate attack in hydrated Portland-limestone cement assessed by 29Si NMR spectroscopy and thermal analysis, Mater. Today: Proc. (2023) in press; DOI: 10.1016/j.matpr.2023.06.185.
- E. R. Chakalov, R. P. Shekurov, V. A. Miluykov, P. M. Tolstoy, Evidence of extremely short hydrogen bond in homoconjugated anion of ferrocene-1,1’-diyl-bisphosphinic acid: sign change of H/D isotope effect on the 31P NMR chemical shift, Phys. Chem. Chem. Phys. (2023) in press; DOI: 10.1039/D3CP03714B.
- E.S. Starnovskaya, M.I. Savchuk, D.S. Kopchuk, O.S. Taniya, A.F. Khasanov, A.S. Novikov, N.V. Slovesnova, A.S. Minin, S. Santra, G.V. Zyryanov, 2-(Indol-3-yl)- and 6-(pyrrol-2-yl)-substituted (bi)pyridine-based AIE-probes/fluorophores: Synthesis and photophysical studies, New J. Chem. (2023) In press.; DOI: 10.1039/D3NJ04051H.
- S. Roy, S. Mal, R. Banik, S. Das, Ľ. Dlháň, J. Titiš, R. Boča, A.M. Kirillov, A.S. Novikov, P. Hazendonk, R.J. Butcher, A. Bauza, A. Frontera, Two isostructural complexes of Ni(II) and Zn(II) with violurate and pyridine: a detailed structural, theoretical, magnetic, and NMR investigation, CrystEngComm (2023) In press.; DOI: 10.1039/d3ce00871a.
- Y. Kenzhebayeva, I. Gorbunova, A. Dolgopolov, M.V. Dmitriev, T.S. Atabaev, E.A. Stepanidenko, A.S. Efimova, A.S. Novikov, S. Shipilovskikh, V.A. Milichko, Self-Assembly of Hydrogen-Bonded Organic Crystals on Arbitrary Surfaces for Efficient Amplified Spontaneous Emission, Adv. Photonics Res. (2023) In press.; DOI: 10.1002/adpr.202300173.
- V.G. Popova, L.V. Kulik, R.I. Samoilova, D.V. Stass, V.V. Kokovkin, E.M. Glebov, A.S. Berezin, A.S. Novikov, A. Garcia, H.T. Tuan, R.D. Rodriguez, M.N. Sokolov, P.A. Abramov, Noncovalent Dualism in Perylene-Diimide-Based Keggin Anion Complexes: Theoretical and Experimental studies, Inorg. Chem. (2023) In press.; DOI: 10.1021/acs.inorgchem.3c03030.
- P. Debnath, C. Majumder, A. Bhattacharya, P. Debnath, S. Roy, A.S. Novikov, M. Roy, T.K. Misra, Crystal Structure of 2-((E)-((Z)-3-(((4-hydroxyphenyl)amino)methylene)-4-oxocyclohexa-1,5-dien-1-yl)diazenyl)benzoic Acid and Synthesis, Spectroscopy, and DFT Study of Its Dibutyltin(IV) Complex, J. Chem. Crystallogr. (2023) In press. DOI: 10.1007/s10870-023-00996-y.
- E. I. Chikunova, V. Yu. Kukushkin, A. Yu. Dubovtsev, Non-Friedländer Route to Diversely 3‑Substituted Quinolines through Au(III)-Catalyzed Annulation Involving Electron-Deficient Alkynes, Org. Lett., 25 (2023); doi: 10.1021/acs.orglett.3c03775.
- A. A. Sysoeva, A. S. Novikov, V. V. Suslonov, D. S. Bolotin, M. V. Il'in, 2,1,3-Benzoselenadiazole-containing zinc(II) halide complexes: chalcogen bonding in the solid state and catalytic activity in the Schiff condensation, Inorg. Chim. Acta 561 (2024) 121867. DOI: 10.1016/j.ica.2023.121867.
- I. N. Klyukin, A. V. Kolbunova, A. S. Novikov, K. Yu. Zhizhin, N. T. Kuznetsov, Theoretical investigation of CB5H6(Hfac) and CB5H5(H2) carboranes: combined MO and QTAIM analysis, Comput. Theor. Chem. 1231 (2024) 114434 DOI: 10.1016/j.comptc.2023.114434.
- A. V. Nelyubin, N. K. Neumolotov, N. A. Selivanov, A. Y. Bykov, I. N. Klyukin, A. S. Novikov, A. S. Kubasov, A. P. Zhdanov, K. Y. Zhizhin, N. T. Kuznetsov, Reduction of triple bond in [B12H11NCR]− anions by lithium aluminum hydride: a novel approach to the synthesis of N-monoalkylammonio-substituted closo-dodecaborates, Inorganics 12 (2024) 2. DOI: 10.3390/inorganics12010002.
- A. Yu. Samsonova, A. Yu. Mikheleva, K. M. Bulanin, N. I. Selivanov, A. S. Mazur, P. M. Tolstoy, C. C. Stoumpos, Y. V. Kapitonov, Internal Vibrations of Pyridinium Cation in One-Dimensional Halide Perovskites and the Corresponding Halide Salts, Molecules, 29 (2024) 78. DOI: 10.3390/molecules29010078.
See also
- A. V. Artem'ev, U. A. Kuzmina, A. Yu. Baranov, A. S. Novikov, I. Yu. Bagryanskaya, Ag(I), Au(I) and Au(I)-Ag(I) clusters based on tris[(6-methylpyridin-2-yl)methyl]phosphine, Inorg. Chem. Commun. 161 (2024) 112131. DOI: 10.1016/j.inoche.2024.112131.
- A. W. Temesgen, A. A. Sapronov, A. S. Kubasov, A. S. Novikov, T. A. Le, A. G. Tskhovrebov, Synthesis and crystal structure of the adduct between 2-pyridylselenyl chloride and isobutyronitrile, Acta Crystallogr. E 80 (2024) E80. DOI: 10.1107/S2056989024000938.
- A. V. Artem'ev, E. H. Sadykov, S. S. Shilo, A. Yu. Baranov, M. I. Rakhmanova, A. S. Novikov, D. G. Samsonenko, I. Yu. Bagryanskaya, 1D, 2D and 3D networks based on group 11 metal ions and (pyrazin-2-ylmethyl)phosphines: synthesis, metallophilic interactions and emission properties, Inorg. Chim. Acta (2024) In press. DOI: 10.1016/j.ica.2024.121964.
- A. S. Zaguzin, A. V. Zaitsev, N. A. Korobeynikov, A. S. Novikov, A. N. Usoltsev, V. P. Fedin, S. A. Adonin, 4,5-diiodo-1-H-imidazole-derived linker ligand and Cu(I) and Co(II) coordination polymers based thereupon, CrystEngComm (2024) In press. DOI: 10.1039/D4CE00072B.
- A. Krinochkin, M. Valieva, E. Starnovskaya, N. Slovesnova, A. Minin, A. Belousova, L. Sadieva, O. Taniya, A. Khasanov, A. Novikov, V. Bruskov, S. Vatolina, D. opchuk, P. Slepukhin, V. Sharutin, G. Zyryanov, New Fluorescent Dye for the Detection of Zn2+ in Living Cells and Fixed Sections of the Rat Pancreas, J. Fluoresc. (2024). DOI: 10.1007/s10895-024-03603-1.
- M. V. Il’in, Y. V. Safinskaya, D. A. Polonnikov, A. S. Novikov, D. S. Bolotin, Chalcogen- and Halogen-Bond-Donating Cyanoborohydrides Provide Imine Hydrogenation, J. Org. Chem. (2024) In press. DOI: 10.1021/acs.joc.3c02282.
- Y. V. Demyanov, T. S. Sukhikh, I. Yu. Bagryanskaya, A. S. Novikov, M. I. Rakhmanova, A. V. Artem'ev, Homo- and heterometallic complexes designed on group 11 metals and tris(6-methyl-2-pyridyl)phosphine: Synthesis, metallophilic interactions and room-temperature phosphorescence, Polyhedron 252 (2024) 116901. DOI: 10.1016/j.poly.2024.116901.
- R. Ninayan, U. Markova, E. Nizov, M. Melesova, A. S. Novikov, A. Shishov, Deep eutectic solvents vs. Aqueous acids in metal extraction from animal tissues, Microchem. J., 200 (2024) 110252. DOI: 10.1016/j.microc.2024.110252.
- N. A. Korobeynikov, A. N. Usoltsev, M. N. Sokolov, A. S. Novikov, S. A. Adonin, Polymeric polyiodo-chlorotellurates(iv): new supramolecular hybrids in halometalate chemistry, CrystEngComm (2024) In press. DOI: 10.1039/D4CE00088A.
- R. Ninayan, U. Markova, E. Nizov, M. Melesova, A. S. Novikov, A. Shishov, Deep eutectic solvents vs. Aqueous acids in metal extraction from animal tissues, Microchem. J., 200 (2024) 110252. DOI: 10.1016/j.microc.2024.110252.
- N. A. Korobeynikov, A. N. Usoltsev, M. N. Sokolov, A. S. Novikov, S. A. Adonin, Polymeric polyiodo-chlorotellurates(iv): new supramolecular hybrids in halometalate chemistry, CrystEngComm (2024) In press. DOI: 10.1039/D4CE00088A.
- A.S. Novikov, M.V. Il’in, Computational Study on the Route of Cooperative Organocatalysis Utilizing Thiourea and Halogen Bond Donor Mixture, Russ. J. Gen. Chem. 94 (Suppl 1) (2024) S129. DOI: 10.1134/S1070363224140135.
- E.A. Dukhnovsky, A.S. Novikov, A.S. Kubasov, A.V. Borisov, N.D. Sikaona, A.A. Kirichuk, V.N. Khrustalev, A.S. Kritchenkov, A.G. Tskhovrebov, Halogen Bond-Assisted Supramolecular Dimerization of Pyridinium-Fused 1,2,4-Selenadiazoles via Four-Center Se2N2 Chalcogen Bonding, Int. J. Mol. Sci. 25 (2024) 3972. DOI: 10.3390/ijms25073972.
- A.E. Kopotilova, M.I. Valieva, E.A. Kudryashova, E.S. Starnovskaya, T.N. Moshkina, E.V. Nosova, O.S. Taniya, A.A. Kalinichev, A.S. Novikov, D.S. Kopchuk, P.A. Slepukhin, V.N. Charushin, Methyl- and Methoxy-substituted 2-(Pyridin-2-yl)-4-(4-aminophenyl)quinazolines: Synthesis and Photophysical Properties, Asian J. Org. Chem. (2024) In press. DOI: 10.1002/ajoc.202400135.
See also
2021
- A. S. Mikherdov, R. A. Popov, M. A. Kinzhalov, M. Haukka, V. A. Polukeev, V. P. Boyarskiy, A. Roodt, Reaction mechanism of regioisomerization in binuclear (diaminocarbene)PdII complexes, Inorg. Chim. Acta, 514 (2021), 120012. DOI: 10.1016/j.ica.2020.120012.
- R. A. Popov, A. S. Mikherdov, A. S. Novikov, L. V. Myznikov, V. P. Boyarskiy, PdII- and PtII-mediated coupling of aryl isocyanides with N-heterocyclic thiones, New J. Chem., 45 (2021), Advance Article. DOI: 10.1039/D0NJ05386D.
- D. P. Zimin, D. V. Dar’in, V. Yu. Kukushkin, A. Yu. Dubovtsev, Oxygen atom transfer as key to reverse chemoselectivity in the gold(I)-catalyzed generation of aminooxazoles from ynamides, J. Org. Chem., 86 (2021); doi: 10.1021/acs.joc.0c02584.
- E. A. Katlenok, A. V. Rozhkov, O. V. Levin, M. Haukka, M. L. Kuznetsov, V. Yu. Kukushkin, Halogen bonding involving palladium(II) as an XB acceptor, Crystal Growth & Design, 21 (2021); doi: 10.1021/acs.cgd.0c01474.
- K. V. Deriabin, N. A. Ignatova, S. O. Kirichenko, A. S. Novikov, R. M. Islamova, Nickel(II)-pyridinedicarboxamide-co-polydimethylsiloxane complexes as elastic self-healing silicone materials with reversible coordination, Polymer, (2020) In press.; DOI: 10.1016/j.polymer.2020.123119.
- M. A. Bondarenko, M. I. Rakhmanova, P. E. Plyusnin, P. A. Abramov, A. S. Novikov, K. Rajakumar, M. N. Sokolov, S. A. Adonin, Heteroleptic Zn(II) 3,5-diiodosalicylates: structures, luminescence and features of non-covalent interactions in solid state, Polyhedron, (2020) In press.; DOI: 10.1016/j.poly.2020.114895.
- I. S. Aliyarova, D. M. Ivanov, N. S. Soldatova, A. S. Novikov, P. S. Postnikov, M. S. Yusubov, V. Yu. Kukushkin, Bifurcated halogen bonding involving diaryliodonium cations as iodine(III)-based double sigma-hole donors, Crystal Growth & Design, 21 (2021); doi: 10.1021/acs.cgd.0c01463.
- Z. M. Efimenko, A. A. Eliseeva, D.M. Ivanov, B. Galmés, A. Frontera, N. A. Bokach, V. Yu. Kukushkin, Bifurcated m2-I···(N,O) halogen bonding: The case of (Nitrosoguanidinate)NiII co-crystals with iodine(I)-based sigma-hole donors, Crystal Growth & Design, 20 (2020); doi: 10.1021/acs.cgd.0c01408.
- M. Bulatova, D. M. Ivanov, M. Haukka, Classics Meet Classics: Theoretical and Experimental Studies of Halogen Bonding in Adducts of Platinum(II) 1,5-Cyclooctadiene Halide Complexes with Diiodine, Iodoform, and 1,4-Diiodotetrafluorobenzene, Crystal Growth & Design, 21 (2021); doi: 10.1021/acs.cgd.0c01314.
- M. A. Bondarenko, A. S. Novikov, P. A. Abramov, I. F. Sakhapov, M. N. Sokolov, S. A. Adonin, 2,3,4,5-Tetraiodopyrrole as a building block for halogen bonding: formation of supramolecular hybrids with organic iodide salts in solid state, J. Mol. Struct., 1230 (2021) 129931. DOI: 10.1016/j.molstruc.2021.129931.
- V. V. Voinova, N. A. Selivanov, I. V. Plyushchenko, M. F. Vokuev, A. Y. Bykov, I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, M. S. Grigoriev, I. A. Rodin, K. Y. Zhizhin, N. T. Kuznetsov, Fused 1,2-diboraoxazoles based on closo-decaborate anion–novel members of diboroheterocycle class, Molecules, 26 (2021) 248. DOI: 10.3390/molecules26010248.
- S. N. Yunusova, A. S. Novikov, N. S. Soldatova, M. A. Vovk, D. S. Bolotin, Iodonium salts as efficient iodine(iii)-based noncovalent organocatalysts for Knorr-type reactions, RSC Adv., 11 (2021) 4574–4583. DOI: 10.1039/d0ra09640g.
- M. V. Dobrynin, E. V. Sokolova, M. A. Kinzhalov, A. S. Smirnov, G. L. Starova, V. Yu. Kukushkin, R. M. Islamova, Cyclometalated platinum(II) complexes simultaneously сatalyze the cross-linking of polysiloxanes and function as luminophores, ACS Applied Polymer Materials, 2021; doi: 10.1021/acsapm.0c01190.
- S. A. Katkova, K. V. Luzyanin, A. S. Novikov, M. A. Kinzhalov, Modulation of luminescence properties for [cyclometalated]-PtII(isocyanide) complexes upon co-crystallisation with halosubstituted perfluorinated arenes, New J. Chem., (2021) In press. DOI: 10.1039/D0NJ05457G.
- A. N. Usoltsev, T. S. Sukhikh, A. S. Novikov, V. R. Shayapov, D. P. Pishchur, I. V. Korolkov, I. F. Sakhapov, V. P. Fedin, M. N. Sokolov, S. A. Adonin, Unexpected polymorphism in bromoantimonate(III) complexes and its effect on optical properties, Inorg. Chem., (2021) In press. DOI: 10.1021/acs.inorgchem.0c03699.
- F. Jiménez-Grávalos, M. Gallegos, Á. M. Pendás, A. S. Novikov, Challenging the electrostatic σ-hole picture of halogen bonding using minimal models and the interacting quantum atoms approach, J. Comput. Chem., (2021) In press. DOI: 10.1002/jcc.26488.
- A. A. Eliseeva, D. M. Ivanov, A. V. Rozhkov, I. V. Ananyev, A. Frontera, V. Yu. Kukushkin, Bifurcated halogen bonding involving two rhodium(I) centers as an integrated σ-hole acceptor, JACS Au, 1 (2021) 354-361. DOI: 10.1021/jacsau.1c00012.
- A. S. Novikov, IsoStar program suite for studies of noncovalent interactions in crystals of chemical compounds, Crystals, 11 (2021) 162. DOI: 10.3390/cryst11020162.
- S. V. Baykov, S. I. Presnukhina, A. S. Novikov, A. A. Shetnev, V. P. Boyarskiy, V. Yu. Kukushkin, 2,5-Dibromothiophenes: halogen bond involving packing patterns and their relevance to solid-state polymerization, Crystal Growth & Design, 21 (2021); doi: 10.1021/acs.cgd.1c00184.
- L. E. Zelenkov, A. A. Eliseeva, S. V. Baykov, V. V. Suslonov, B. Galmés, A. Frontera, V. Yu. Kukushkin, D. M. Ivanov, N. A. Bokach, Electron belt-to-σ-Hole switch of noncovalently bound iodine(I) atoms in dithiocarbamate metal complexes, Inorg. Chem. Front., 2021; doi: 10.1039/d1qi00314c.
- M.A. Bondarenko, A.S. Novikov, I.F. Sakhapov, M.N. Sokolov, S.A. Adonin, Heteroleptic Cu(I) halide complexes with perchlorinated 1,10-phenanthroline, J. Mol. Struct., 1234 (2021) 130199. DOI: 10.1016/j.molstruc.2021.130199.
- I.N. Klyukin, Y.S. Vlasova, A.S. Novikov, A.P. Zhdanov, K.Y. Zhizhin, N.T. Kuznetsov, Theoretical study of closo-borate anions [BnHn]2− (n = 5–12): bonding, atomic charges, and reactivity analysis, Symmetry, 13 (2021) 464. DOI: 10.3390/sym13030464.
- A.N. Usoltsev, N.A. Korobeynikov, B.A. Kolesov, A.S. Novikov, D.G. Samsonenko, V.P. Fedin, M.N. Sokolov, S.A. Adonin, Rule, not exclusion: formation of dichlorine-containing supramolecular complexes with chlorometalates(IV), Inorg. Chem., 60 (2021) 4171. DOI: 10.1021/acs.inorgchem.1c00436.
- S. V. Baykov, K. K. Geyl, D. M. Ivanov, R. M. Gomila, A. Frontera, V. Yu. Kukushkin, Azine steric hindrances switch halogen bonding to N-arylation upon interplay with σ-hole donating haloarenenitriles, Chem. Asian J., 16 (2021); doi: 10.1002/asia.202100282.
- V.V. Voinova, I.N. Klyukin, A.S. Novikov, A.Ya. Koz’menkova, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, Electrochemical properties of the closo-decaborate anion [B10H10]2– and a new method for preparation of the [B20H18]2– anion, Russ. J. Inorg. Chem., 66 (2021) 295. DOI: 10.1134/S0036023621030190.
- N.G. Shikhaliyev, A.M. Maharramov, K.N. Bagirova, G.T. Suleymanova, B.D. Tsyrenova, V.G. Nenajdenko, A.S. Novikov, V.N. Khrustalev, A.G. Tskhovrebov, Supramolecular organic frameworks derived from bromoaryl-substituted dichlorodiazabutadienes via Cl···Br halogen bonding, Mendeleev Commun., 31 (2021) 191. DOI: 10.1016/j.mencom.2021.03.015.
- A.V. Nelyubin, I.N. Klyukin, A.S. Novikov, A.P. Zhdanov, M.S. Grigoriev, K.Yu. Zhizhin, N.T. Kuznetsov, Nucleophilic addition of amino acid esters to nitrilium derivatives of closo-decaborate anion, Mendeleev Commun., 31 (2021) 201. DOI: 10.1016/j.mencom.2021.03.018.
- V.N. Khrustalev, A.O. Savchenko, A.I. Zhukova, N.Yu. Chernikova, M.A. Kurykin, A.S. Novikov, A.G. Tskhovrebov, Attractive fluorine···fluorine interactions between perfluorinated alkyl chains: a case of perfluorinated Cu(II) diiminate Cu[C2F5–C(NH)–CF=C(NH)–CF3]2, Z. Kristallogr. Cryst. Mater., 236 (2021) 117. DOI: 10.1515/zkri-2021-2009.
- A.G. Tskhovrebov, A.S. Novikov, B.S. Tupertsev, A.A. Nazarov, A.A. Antonets, A.A. Astafiev, A.S. Kritchenkov, A.S. Kubasov, V.G. Nenajdenko, V.N. Khrustalev, Azoimidazole gold(III) complexes: synthesis, structural characterization and self-assembly in the solid state, Inorg. Chim. Acta, 522 (2021) 120373. DOI: 10.1016/j.ica.2021.120373.
- M.A. Bondarenko, P.A. Abramov, P.E. Plyusnin, A.S. Novikov, M.N. Sokolov, S.A. Adonin, Bromoantimonates with bis(pyridinium)-type dications obtained via oxidation by dibromine: diverse structural types and features of interactions pattern, Polyhedron, (2021) In press. DOI: 10.1016/j.poly.2021.115217.
- A. Yu. Dubovtsev, V. V. Zvereva, N. V. Shcherbakov, D. V. Dar’in, A. S. Novikov, V. Yu. Kukushkin, Acid-catalyzed [2+2+2] cycloaddition of two cyanamides and one ynamide: highly regioselective synthesis of 2,4,6-triaminopyrimidines, Org. Biomol. Chem., 2021; doi: 10.1039/D1OB00513H.
- N. V. Shcherbakov, D. V. Dar’in, V. Yu. Kukushkin, A. Yu. Dubovtsev, Hetero-Tetradehydro-Diels–Alder cycloaddition of enynamides and cyanamides: gold-catalyzed generation of diversely substituted 2,6-diaminopyridines, J. Org. Chem., 86 (2021); doi: 10.1021/acs.joc.1c00558.
- A.A. Nikitina, V.A. Milichko, A.S. Novikov, A.O. Larin, P. Nandi, U. Mirsaidov, D.V. Andreeva, M.V. Rybin, Y.S. Kivshar, E.V. Skorb, All-dielectric nanostructures with a thermoresponsible dynamic polymer shell, Angew. Chem. Int. Ed., (2021) In press. DOI: 10.1002/anie.202101188.
- A.N. Usoltsev, N.A. Korobeynikov, B.A. Kolesov, A.S. Novikov, P.A. Abramov, M.N. Sokolov, S.A. Adonin, Oxochloroselenate(IV) with Incorporated {Cl2}: The Case of Strong Cl···Cl Halogen Bonding, Chem. Eur. J., (2021) In press. DOI: 10.1002/chem.202101024.
- A.A. Mukhacheva, V.Yu. Komarov, V.V. Kokovkin, A.S. Novikov, P.A. Abramov, M.N. Sokolov, Unusual π-π interactions directed by [{(C6H6)Ru}2W8O30(OH)2]6– hybrid anion, CrystEngComm, (2021) In press. DOI: 10.1039/D1CE00319D.
- M.A. Bondarenko, A.S. Novikov, I.V. Korolkov, M.N. Sokolov, S.A. Adonin, Cu(II) 2-iodobenzoates: precursor-dependent formation of paddlewheel-like [Cu2(OOCR)4L2] or [Cu2L4(OOCR)2Cl2] binuclear complexes, Inorg. Chim. Acta, (2021) In press. DOI: 10.1016/j.ica.2021.120436.
- S. V. Baykov, A. V. Semenov, E. A. Katlenok , A. A. Shetnev, N. A. Bokach, Comparative structural study of three tetrahalophthalic anhydrides: recognition of X···O(anhydride) halogen bond and πh···O(anhydride) interaction, Molecules, 26 (2021) 3119; DOI: 10.3390/molecules26113119.
- V. P. Boyarskii, A. S. Mikherdov, S. V. Baikov, P. Yu. Savko, R. V. Suezov, R. E. Trifonov, Diaminocarbene Complexes of Palladium(II) Containing 2-Aminooxazole and 2-Aminothiazole Heterocyclic Ligands as Potential Antitumor Agents. Pharm. Chem. J. 55 (2021), 130–132. DOI: 10.1007/s11094-021-02393-1.
- V. V. Matveevskaya, D. I. Pavlov, D. G. Samsonenko, E. A. Ermakova, L. S. Klyushova, S. V. Baykov, V. P. Boyarskiy, A. S. Potapov, Synthesis and Structural Characterization of Half-Sandwich Arene–Ruthenium(II) Complexes with Bis(Imidazol-1-Yl)Methane, Imidazole and Benzimidazole. Inorganics 9 (2021), 34. DOI: 10.3390/inorganics9050034.
- N. Soldatova, A. Semenov, K. Geyl, S. Baykov, A. Shetnev, A. Konstantinova, M. Korsakov, M. Yusubov, P. Postnikov, Copper‐Catalyzed Selective N‐Arylation of Oxadiazolones by Diaryliodonium Salts. Adv. Synth. Catal. (2021) In press. DOI: 10.1002/adsc.202100426.
- A. V. Rozhkov, I. V. Ananyev, A. A. Petrov, B. Galmés, A. Frontera, N. A. Bokach, V. Yu. Kukushkin, Ligand steric hindrances switch bridging (μ2-I)···O,O to two-center I···O halogen bonding mode in the assembly of diketonate copper(II) species, Crystal Growth & Design, 21 (2021); doi: 10.1021/acs.cgd.1c00373.
- S. Kasatkina, K. Geyl, S. Baykov, I. A. Boyarskaya, V. Boyarskiy, Catalyst-free synthesis of substituted pyridin-2-yl, quinolin-2-yl, and isoquinolin-1-yl carbamates from the corresponding hetaryl ureas and alcohols. Org. Biomol. Chem. (2021) In press. DOI: 10.1039/D1OB00783A.
- V. Rostovtseva, A. Pulyalina, R. Dubovenko, I. Faykov, K. Subbotina, N. Saprykina, A. Novikov, L. Vinogradova, G. Polotskaya, Enhancing pervaporation membrane selectivity by incorporating star macromolecules modified with ionic liquid for intensification of lactic acid dehydration, Polymers, 13 (2021) 1811. DOI: 10.3390/polym13111811.
- M.A. Vershinin, M.I. Rakhmanova, A.S. Novikov, M.N. Sokolov, S.A. Adonin, Zn(II) heteroleptic halide complexes with 2-halopyridines: features of halogen bonding in solid state, Molecules, 26 (2021) 3393. DOI: 10.3390/molecules26113393.
- O.V. Rudnitskaya, T.A. Tereshina, E.V. Dobrokhotova, E.K. Kultyshkina, A.S. Novikov, A.G. Tskhovrebov, Y.V. Zubavichus, V.N. Khrustalev, Monoprotonated dimethyl sulfoxide, [HOSMe2]+: synthesis, crystal structure, spectroscopic and theoretical studies of [HOSMe2]2[OsCl6] ⋅ 2H2O, ChemistrySelect, 6 (2021) 5211. DOI: 10.1002/slct.202100970.
- V.N. Khrustalev, M.M. Grishina, Z.V. Matsulevich, J.M. Lukiyanova, G.N. Borisova, V.K. Osmanov, A.S. Novikov, A.A. Kirichuk, E. Solari, A.V. Borisov, A.G. Tskhovrebov, Novel cationic 1,2,4-selenadiazoles: synthesis via addition of 2-pyridylselenyl halides to unactivated nitriles, structures and four-center Se···N contacts, Dalton Trans., (2021) In press. DOI: 10.1039/D1DT01322J.
- V.A. Dodonov, O.A. Kushnerova, E.V. Baranov, A.S. Novikov, I.L. Fedushkin, Activation and modification of carbon dioxide by redox-active low-valent gallium species, Dalton Trans., (2021) In press. DOI: 10.1039/D1DT01199E.
- A.S. Novikov, A.L. Gushchin, Trinuclear molybdenum clusters with sulfide bridges as potential anionic receptors via chalcogen bonding, CrystEngComm, (2021) In press. DOI: 10.1039/D1CE00514F.
- K. V. Deriabin, N. A. Ignatova, S. O. Kirichenko, A. S. Novikov, M. A. Кryukova, V. Yu. Kukushkin, R. M. Islamova, Structural features of polymer ligand environments dramatically affect the mechanical and room-temperature self-healing properties of cobalt(II)-incorporating polysiloxanes, Organometallics, 2021; doi: 10.1021/acs.organomet.1c00392.
- M.A. Bondarenko, A.S. Novikov, T.S. Sukhikh, I.V. Korolkov, M.N. Sokolov, S.A. Adonin, Mono- and binuclear Cu (II) 3,5-diiodosalicylates: Structures and features of non-covalent interactions in crystalline state, J. Mol. Struct., 1244 (2021) 130942. DOI: 10.1016/j.molstruc.2021.130942.
- M.V. Kashina, D. M. Ivanov, M. A. Kinzhalov, The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds, Crystals, 11 (2021) 799–901; DOI: 10.3390/cryst11070799.
- V. Suslonov, N. S. Soldatova, D. M. Ivanov, B. Galmés, A. Frontera, G. Resnati, P. S. Postnikov, V. Yu. Kukushkin, N. A. Bokach, Diaryliodonium tetrachloroplatinates(II): recognition of a trifurcated metal-involving μ3-I∙∙∙(Cl,Cl,Pt) halogen bond, Crystal Growth & Design, 21 (2021); doi: 10.1021/acs.cgd.1c00654.
- A. A. Shetnev, V. A. Panova, P. M. Kutuzova, M. V. Tarasenko, M. V. Zhmykhova, S. V. Baykov, S. I. Filimonov, Synthesis and Photoluminescent Properties of 2-(3-Carboxymethylindazol-1-yl)anilines. Russ. J. Gen. Chem. 91 (2021), 985–990. DOI: 10.1134/S1070363221060049.
- M. V. Tarasenko, V. D. Kotlyarova, S. V. Baykov, A. A. Shetnev, 2-(1,2,4-Oxadiazol-5-yl)anilines Based on Amidoximes and Isatoic Anhydrides: Synthesis and Structure Features. Russ. J. Gen. Chem. 91 (2021), 768–778. DOI: 10.1134/S1070363221050030.
- A. M. Cheranyova, D. M. Ivanov, Tetrabromoethane as σ-Hole Donor toward Bromide Ligands: Halogen Bonding between C2H2Br4 and Bromide Dialkylcyanamide Platinum(II) Complexes, Crystals, 11 (2021) 835; DOI: 10.3390/cryst11070835.
- M. Bulatova, D. M. Ivanov, J. M. Rautiainen, M. A. Kinzhalov , K.-N. Truong, M. Lahtinen, M. Haukka, Studies of Nature of Uncommon Bifurcated I–I···(I–M) Metal-Involving Noncovalent Interaction in Palladium(II) and Platinum(II) Isocyanide Cocrystals, Inorg. Chem., 60 (2021) 13200-13211. DOI: 10.1021/acs.inorgchem.1c01591.
- P. A. Abramov, A.S. Novikov, M.N. Sokolov, Interactions of aromatic rings in the crystal structures of hybrid polyoxometalates and Ru clusters, CrystEngComm, (2021) In press. DOI: 10.1039/D1CE00716E.
- N. V. Shcherbakov, D. V. Dar’in, V. Yu. Kukushkin, A. Yu. Dubovtsev, Gold-сatalyzed nitrene transfer from benzofuroxans to N-allylynamides: synthesis of 3-azabicyclo[3.1.0]hexanes, J. Org. Chem., 86 (2021). doi: 10.1021/acs.joc.1c01654.
- A. V. Rozhkov, E. A. Katlenok, M. V. Zhmykhova, A. Yu. Ivanov, M. L. Kuznetsov, N. A. Bokach, V. Yu. Kukushkin, Metal-involving chalcogen bond. The case of platinum(II) interaction with Se/Te-based sigma-hole donors, J. Am. Chem. Soc., 143 (2021). doi: 10.1021/jacs.1c06498.
- A.A. Sysoeva, A.S. Novikov, M.V. Il'in, V.V. Suslonov, D.S. Bolotin, Predicting the catalytic activity of azolium-based halogen bond donors: an experimentally-verified theoretical study. Org. Biomol. Chem., 2021, 19, 7611–7620. DOI: 10.1039/D1OB01158H.
- N. Y. Shmelev, T. H. Okubazghi, P. A. Abramov, V. Y. Komarov, M. I. Rakhmanova, A. S. Novikov, A. L. Gushchin, Intramolecular aurophilic interactions in dinuclear gold(I) complexes with twisted bridging 2,2′-bipyridine ligands, Dalton Trans., 50 (2021) 12448-12456; DOI: 10.1039/d1dt02164h.
- M. A. Bondarenko, A. S. Novikov, K. V. Chernova, M. N. Sokolov, S. A. Adonin, 2-Methylpyridinium salt of pentaiodobenzoic acid: role of the halogen bond in the formation of a crystal packing, J. Struct. Chem., 62 (2021) 1237-1242; DOI: 10.1134/S0022476621080096.
- A. N. Lukoyanov, I. S. Fomenko, M. I. Gongola, L. S. Shul’pina, N. S. Ikonnikov, G. B. Shul’pin, S. Y. Ketkov, G. K. Fukin, R. V. Rumyantcev, A. S. Novikov, V. A. Nadolinny, M. N. Sokolov, A. L. Gushchin, Novel oxidovanadium complexes with redox-active R-mian and R-bian ligands: synthesis, structure, redox and catalytic properties, Molecules, 26 (2021) 5706; DOI: 10.3390/molecules26185706.
- Z. M. Efimenko, A. V. Rozhkov, V. V. Suslonov, M. L. Kuznetsov, V. Y. Kukushkin, N. A. Bokach, Copper(II)-mediated iodination of 1-nitroso-2-naphthol, Molecules, 26 (2021) 5708; DOI: 10.3390/molecules26185708.
- Y. N. Toikka, D. V. Spiridonova, A. S. Novikov, N. A. Bokach, Copper(II) prevents the saccarine-dialkylcyanamide coupling by forming mononuclear (saccharinate)(dialkylcyanamide)copper(II) complexes, Inorganics, 9 (2021) 69; DOI: 10.3390/inorganics9090069.
- S. V. Baykov, A. S. Mikherdov, A. S. Novikov, K. K. Geyl, M. V. Tarasenko, M. A. Gureev, V. P. Boyarskiy, π–π Noncovalent Interaction Involving 1,2,4- and 1,3,4-Oxadiazole Systems: The Combined Experimental, Theoretical, and Database Study, Molecules, 26 (2021), 5672. DOI: 10.3390/molecules26185672.
- A. S. Miroshnichenko, K. V. Deryabin, M. Baeva, F. M. Kochetkov, V. Neplokh, V. V. Fedorov, D. V. Krasnikov, V. A. Sharov, N. A. Filtov, D. S. Gets, S. V. Makarov, A. G. Nasibulin, I. S. Mukhin, V. Yu. Kukushkin, R. M. Islamova, Flexible perovskite CsPbBr3 Light Emitting Devices integrated with gap nanowire arrays in highly transparent and durable functionalized silicones, J. Phys. Chem. Lett., 12 (2021) 9672-9676. DOI: 10.1021/acs.jpclett.1c02611.
- Yu. V. Torubaev, I. V. Skabitsky, A. V. Rozhkov, B. Galmés, A. Frontera, V. Yu. Kukushkin, Highly polar stacking interactions wrap inorganics in organics: Lone-pair–pi-hole interactions between the PdO4 core and electron-deficient arenes, Inorg. Chem. Front., 8 (2021) 4965-4975. DOI: 10.1039/D1QI01067K.
- M. V Dobrynin, S. Kasatkina, S. Baykov, P. Savko, N. Antonov, A. S. Mikherdov, V. Boyarskiy, R. M. Islamov, Deprotonated diaminocarbene platinum complexes for thermoresponsive luminescent silicone materials: both catalysts and luminophores, Dalton Trans., 50 (2021) 14994-14999. DOI: 10.1039/D1DT02823E.
- N. V. Shcherbakov, D. V. Dar’in, V. Yu. Kukushkin, A. Yu. Dubovtsev, Redox-neutral synthesis of β-carbolines via gold-сatalyzed [4+2] cycloaddition of indolylynamides and cyanamides, J. Org. Chem., 86 (2021) 17804-17815. DOI: 10.1021/acs.joc.1c02119.
- I. S. Giba, P. M. Tolstoy, Self-assembly of tetrahedral hydrogen-bonded cage tetramers of phosphonic acid, Symmetry 13 (2021) 258; DOI: 10.3390/sym13020258.
- E. Yu. Tupikina, P. M. Tolstoy, A. A. Titova, M. A. Kostin, G. S. Denisov, Estimations of FH···X hydrogen bond energies from IR intensities: Iogansen's rule revisited, J. Comput. Chem. 42 (2021) 564–571; DOI: 10.1002/jcc.26482.
- I. S. Giba, V. V. Mulloyarova, G. S. Denisov, P. M. Tolstoy, Sensitivity of 31P NMR chemical shifts to hydrogen bond geometry and molecular conformation for complexes of phosphinic acids with pyridines, Magn. Reson. Chem. 59 (2021) 465–477; DOI: 10.1002/mrc.5123.
- V. V. Mulloyarova, A. M. Puzyk, A. A. Efimova, A. S. Antonov, R. A. Evarestov, I. S. Aliyarova, R. E. Asfin, P. M. Tolstoy, Solid-state and solution-state self-association of dimethylarsinic acid: IR, NMR and theoretical study, J. Mol. Struct. 1234 (2021) 130176; DOI: 10.1016/j.molstruc.2021.130176.
- V. A. Rozentsvet, N. A. Sablina, D. M. Ulyanova, S. N. Smirnov, P. M. Tolstoy, Structure of the polymer chain of poly(1,3-pentadiene) synthesized using a stereospecific catalytic system, Russ. Chem. Bull. 70 (2021) 773–779; DOI: 10.1007/s11172-021-3150-2.
- B. Koeppe, J. Guo, P. M. Tolstoy, H.-H. Limbach, Combined NMR and UV-Vis spectroscopic studies of models for the hydrogen bond system in the active site of photoactive yellow protein: H-bond cooperativity and medium effects, J. Phys. Chem. B 125 (2021) 5874–5884; DOI: 10.1021/acs.jpcb.0c09923.
- Ł. Hetmańczyk, P. Szklarz, A. Kwocz, M. Wierzejewska, M. Pagacz-Kostrzewa, M. Ya. Melnikov, P. M. Tolstoy, A. Filarowski, Polymorphism and conformational equilibrium for nitro-acetophenone in the solid state and under the matrix condition, Molecules 26 (2021) 3109; DOI: 10.3390/molecules26113109.
- E. Yu. Tupikina, V. V. Karpov, P. M. Tolstoy, On the influence of water molecules on the outer electronic shells of R–SeH, R–Se(–) and R–SeOH fragments in selenocysteine amino acid residue, Phys. Chem. Chem. Phys. 23 (2021) 13965–13970; DOI: 10.1039/D1CP01345A.
- J. Savosina, M. Agafonova-Moroz, M. Khaydukova, A. Legin, V. Babain, P. Tolstoy, D. Kirsanov, On the radiolytic stability of potentiometric sensors with plasticized polymeric membranes, Chemosensors 9 (2021) 214; DOI: 10.3390/chemosensors9080214.
- E. Bulatov, T. Eskelinen, A. Ivanov, P. Tolstoy, E. Kalenius, P. Hirva, M. Haukka, Noncovalent axial I∙∙∙Pt∙∙∙I interactions in platinum(II) complexes strengthen in the excited state, ChemPhysChem 22 (2021) 1–7; DOI: 10.1002/cphc.202100468.
- Ł. Hetmańczyk, E. A. Goremychkin, J. Waliszewski, M. V. Vener, P. Lipkowski, P. M. Tolstoy, A. Filarowski, Spectroscopic identification of hydrogen bond vibrations and quasi-isostructural polymorphism in N-salicylideneaniline, Molecules 26 (2021) 5043; DOI: 10.3390/molecules26165043.
- V. Karpov, A. Puzyk, P. Tolstoy, E. Tupikina, Hydration of selenolate moiety: ab initio investigation of properties of O–H···Se(–) hydrogen bonds in CH3Se(–)∙∙∙(H2O)n clusters, J. Comput. Chem. 42 (2021) 2014–2023; DOI: 10.1002/jcc.26733.
- V. A. Rozentsvet, N. A. Sablina, D. M. Ulyanova, P. M. Tolstoy, I. A. Novakov, Polymerization of isoprene using cationic catalytic systems based on triethylaluminum, Doklady Phys. Chem. 499 (2021) 66–70; DOI: 10.31857/S2686953521040063.
- N. G. Shikhaliyev, A. M. Maharramov, G. T. Suleymanova, A. A. Babazade, V. G. Nenajdenko, V. N. Khrustalev, A. S. Novikov, A. G. Tskhovrebov, Arylhydrazones of α-keto esters via methanolysis of dichlorodiazabutadienes: synthesis and structural study, Mendeleev Commun., 31 (2021) 677-679; DOI: 10.1016/j.mencom.2021.09.028.
- I. N. Klyukin, Y. S. Vlasov, A. S. Novikov, A. P. Zhdanov, H. R. Hagemann, K. Yu. Zhizhin, N. T. Kuznetsov, B-F bonding and reactivity analysis of mono- and perfluoro-substituted derivatives of closo-borate anions (6, 10, 12): A computational study, Polyhedron, 211 (2022) 115559; DOI: 10.1016/j.poly.2021.115559.
- M. M. Efremova, A. A. Makarova, A. S. Novikov, M. A. Kryukova, M. A. Kuznetsov, A. P. Molchanov, Regio- and stereoselective (3 + 2)-cycloaddition reactions of nitrones with cyclic allenes, Org. Biomol. Chem., 19 (2021) 9773-9784; DOI: 10.1039/D1OB01584B.
- A. V. Pomogaeva, O. V. Khoroshilova, E. I. Davydova, V. V. Suslonov, A. Yu. Timoshkin, Antimony(III) Iodide Complexes with Pyridine: Structures and bonding via three pnictogen bonds Z. Anorg. Allg. Chem., 647 (2021) 687–695; doi: 10.1002/zaac.202000444.
- V. Lyutoev, T. Shumilova, A. Mazur, P. Tolstoy, NMR spectral characteristics of ultrahigh-pressure high-temperature impact glasses of the giant Kara astrobleme (Pay-Khoy, Russia), Minerals, 11 (2021) 1418; DOI: 10.3390/min11121418.
- V. Rozentsvet, N. Sablina, D. Ulyanova, S. Kostjuk, P. Tolstoy, Structure of terminal units of polybutadiene synthesized via anionic mechanism, Polym. Bull., (2021), accepted. DOI: 10.1007/s00289-021-03549-5.
- A. V. Nelyubin, N. A. Selivanov, A. Y. Bykov, I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, N. Y. Karpechenko, M. S. Grigoriev, K. Y. Zhizhin, N. T. Kuznetsov, Primary amine nucleophilic addition to nitrilium closo-dodecaborate [B12H11NCCH3]−: a simple and effective route to the new BNCT drug design, Int. J. Mol. Sci., 22 (2021) 13391; DOI: 10.3390/ijms222413391.
- I. V. Buslov, A. S. Novikov, V. N. Khrustalev, M. V. Grudova, A. S. Kubasov, Z. V. Matsulevich, A. V. Borisov, J. M. Lukiyanova, M. M. Grishina, A. A. Kirichuk, T. V. Serebryanskaya, A. S. Kritchenkov, A. G. Tskhovrebov, 2-Pyridylselenenyl versus 2-pyridyltellurenyl halides: symmetrical chalcogen bonding in the solid state and reactivity towards nitriles, Symmetry, 13 (2021) 2350; DOI: 10.3390/sym13122350.
- M. A. Kinzhalov, K. V. Luzyanin, Synthesis and Contemporary Applications of Platinum Group Metals Complexes with Acyclic Diaminocarbene Ligands (Review), Russ. J. Inorg. Chem., 2022, Vol. 67, No. 1, pp. 48–90. DOI: 10.1134/S0036023622010065.
- M. A. Kinzhalov, E. V. Grachova and K. V. Luzyanin, Tuning the Luminescence of Transition Metal Complexes with Acyclic Diaminocarbene Ligands, Inorg. Chem. Front., 2021, accepted. DOI: 10.1039/D1QI01288F.
- A. A. Yakubenko, V. V. Karpov, E. Yu. Tupikina, A. S. Antonov, Lithiation of 2,4,5,7-tetrabromo-1,8-bis(dimethylamino)naphthalene: peculiarities of directing groups’ effects and the possibility of polymetalation, Organometallics, 40 (2021) 3627–3636; DOI: 10.1021/acs.organomet.1c00489.
- V.V. Suslonov, N.S. Soldatova, D.M. Ivanov, B. Galmés, A. Frontera, G. Resnati, P.S. Postnikov, V.Y. Kukushkin, N.A. Bokach, Diaryliodonium Tetrachloroplatinates(II): Recognition of a Trifurcated Metal-Involving μ3-I···(Cl,Cl,Pt) Halogen Bond, Crystal Growth and Design, 21 (2021), 5360-5372; DOI: 10.1021/acs.cgd.1c00654.
- L.E. Zelenkov, A.A. Eliseeva, S.V. Baykov, V.V. Suslonov, B. Galmés, A. Frontera, V.Y. Kukushkin, D.M. Ivanov, N.A. Bokach, Electron belt-to-s-hole switch of noncovalently bound iodine(i) atoms in dithiocarbamate metal complexes, Inorganic Chemistry Frontiers, 8 (2021), 2505-2517; DOI: 10.1039/d1qi00314c.
2020
- N. A. Bokach, D. S. Bolotin, V. Yu. Kukushkin, Nonaromatic nitrogen heterocycles derived from metal-involving reactions of oximes, Chapter 16, in “Synthetic Approaches to Nonaromatic Nitrogen Heterocycles”, Wiley, 2020; accepted for publication.
- N. A. Bokach, D. S. Bolotin, V. Yu. Kukushkin, Metal-catalyzed and metal-mediated reactions of nitrones leading to nonaromatic nitrogen heterocyclic systems, Chapter 17, in “Synthetic Approaches to Nonaromatic Nitrogen Heterocycles”, Wiley, 2020; accepted for publication.
- V. Rozhkov, S. N. Eliseeva, S. Baykov, L. E. Zelenkov, D. Goryachii, I. V. Taydakov, Copper(I) ionic complexes based on imidazo[4,5-f][1,10]phenanthrolin diimine chelating ligands: crystal structures, photo- and electroluminescent properties, New J. Chem., 44 (2020) 110–120; DOI: 10.1039/C9NJ05109K.
- A. A. Eliseeva, D. M. Ivanov, A. S. Novikov, A. V. Rozhkov, I. V. Kornyakov, A. Yu. Dubovtsev, V. Yu. Kukushkin, Hexaiododiplatinate(II) as a useful supramolecular synthon for halogen bond involving crystal engineering, Dalton Trans., 49 (2020) 356–367; DOI: 10.1039/C9DT04221K.
- A.Yu. Dubovtsev, N.V. Shcherbakov, D.V. Dar’in, V.Yu. Kukushkin, Nature of the Nucleophilic Oxygenation Reagent is Key to Acid-Free Gold-Catalyzed Conversion of Terminal and Internal Alkynes to 1,2-Dicarbonyls, J. Org. Chem., 85 (2020) 745–757; DOI: 10.1021/acs.joc.9b02785.
- S. V. Baykov, S. I. Filimonov, A. V. Rozhkov, A. S. Novikov, I. V. Ananyev, D. M. Ivanov, V. Yu. Kukushkin, Reverse Sandwich Structures from Interplay between Lone pair–π-hole Atom-directed C•••dz2[M] and Halogen Bond Interactions, Cryst. Growth Des., (2020) In press; DOI: 10.1021/acs.cgd.9b01334.
- M. V. Il’in, L. A. Lesnikova, D. S. Bolotin, A. S. Novikov, V. V. Suslonov, V. Yu. Kukushkin, One-pot Route to N-acyl Ureas: a Formal Four-Component Hydrolytic Reaction Involving Aminonitrones and Isocyanide Dibromides, New. J. Chem., 44 (2020), 1253-1262; DOI: 10.1039/C9NJ05445F.
- A.V. Chupina, V.R. Shayapov, A.S. Novikov, V.V. Volchek, E. Benassi, P.A. Abramov, M.N. Sokolov, [{AgL}2Mo8O26]n– complexes: combined experimental and theoretical study, Dalton Trans., (2020) In press; DOI: 10.1039/C9DT04043A.
- V. Rozhkov, D. M. Ivanov, A. S. Novikov, I. V. Ananyev, N. A. Bokach, V. Yu. Kukushkin, Metal-involving halogen bond Ar–I∙∙∙[dz2PtII] in the platinum acetylacetonate complex, CrystEngComm, 22 (2020), 554-563; DOI: 10.1039/C9CE01568J.
- S.A. Adonin, M.A. Bondarenko, A.S. Novikov, P.E. Plyusnin, I.V. Korolkov, M.N. Sokolov, V.P. Fedin, Five new Sb(V) bromide complexes and their polybromide derivatives with pyridinium-type cations: structures, thermal stability and features of halogen•••halogen contacts in solid state, Inorg. Chim. Acta, (2020) In press. DOI: 10.1016/j.ica.2019.119278.
- S.A. Adonin, A.S. Novikov, K.V. Chernova, D.A. Vinnik, S.V. Taskaev, I.V. Korolkov, E.V. Ilyina, A.A. Pavlov, V.V. Novikov, M.N. Sokolov, V.P. Fedin, Heteroleptic copper(II) complexes with 2-bromo-5-methylpyridine: structures, features of non-covalent interactions and magnetic behavior, Inorg. Chim. Acta, 502 (2020) 119333; DOI: 10.1016/j.ica.2019.119333.
- O.V. Repina, A.S. Novikov, O.V. Khoroshilova, A.S. Kritchenkov, A.A. Vasin, A.G. Tskhovrebov, Lasagna-like supramolecular polymers derived from the PdII osazone complexes via C(sp2)–H•••Hal hydrogen bonding, Inorg. Chim. Acta, 502 (2020) 119378; DOI: 10.1016/j.ica.2019.119378.
- I.D. Gorokh, S.A. Adonin, A.N. Usoltsev, A.S. Novikov, D.G. Samsonenko, S.V. Zakharov, M.N. Sokolov, V.P. Fedin, Bromide complexes of bismuth with 4-bromobenzyl-substituted cations of pyridinium family, J. Mol. Struct., 1199 (2020) 126955; DOI: 10.1016/j.molstruc.2019.126955.
- E.A. Kostenko, S.V. Baykov, A.S. Novikov, V.P. Boyarskiy, Nucleophilic properties of the positively charged metal center in the solid state structure of palladium(II)-terpyridine complex, J. Mol. Struct., 1199 (2020) 126957; DOI: 10.1016/j.molstruc.2019.126957.
- A.M. Afanasenko, A.S. Novikov, T.G. Chulkova, Y.M. Grigoriev, I.E. Kolesnikov, S.I. Selivanov, G.L. Starova, A.A. Zolotarev, A.N. Vereshchagin, M.N. Elinson, Intermolecular interactions-photophysical properties relationships in phenanthrene-9,10-dicarbonitrile assemblies, J. Mol. Struct., 1199 (2020) 126789; DOI: 10.1016/j.molstruc.2019.07.036.
- K.G. Nikolaev, S.A. Ulasevich, O. Luneva, O.Yu. Orlova, D. Vasileva, S. Vasilev, A.S. Novikov, E.V. Skorb, Humidity-driven transparent holographic free-standing polyelectrolyte films, ACS Appl. Polym. Mater., (2020) In press. DOI: 10.1021/acsapm.9b01151.
- A.S. Mikherdov, S.A. Katkova, A.S. Novikov, M.M. Efremova, E.Yu. Reutskaya, M.A. Kinzhalov, (Isocyano group)•••lone pair interactions involving coordinated isocyanides: experimental, theoretical and CSD study, CrystEngComm, (2020) In press. DOI: 10.1039/C9CE01741K.
- D.S. Bolotin, N.S. Soldatova, M.Y. Demakova, A.S. Novikov, D.M. Ivanov, I.S. Aliyarova, A. Sapegin, M. Krasavin, Pentacoordinated silver(I) complex featuring 8-phenylquinoline ligands: interplay of coordination bonds, semicoordination, and stacking interactions, Inorg. Chim. Acta, (2020) In press. DOI: 10.1016/j.ica.2020.119453.
- V.Y. Mikshiev, A.F. Pozharskii, A. Filarowski, A.S. Novikov, A.S. Antonov, P.M. Tolstoy, M.A. Vovk, O.V. Khoroshilova, How strong is hydrogen bonding to amide nitrogen?, ChemPhysChem, (2020) In press. DOI: 10.1002/cphc.201901104.
- Z. M. Efimenko, A. S. Novikov, D. M. Ivanov, A. V. Piskunov, A. A. Vereshchagin, O. V. Levin, N. A. Bokach, V. Yu. Kukushkin, The (dioximate)NiII/I2 system: ligand oxidation and binding modes of triiodide species, Inorg. Chem., 59 (2020), in press; doi: 10.1021/acs.inorgchem.9b03132.
- A. A. Eremina, M. A. Kinzhalov, E. A. Katlenok, A. S. Smirnov, E. V. Andrusenko, E. A. Pidko, V. V. Suslonov, K. V. Luzyanin, Phosphorescent Iridium(III) Complexes with Acyclic Diaminocarbene Ligands as Chemosensors for Mercury Inorg. Chem., 59 (2020) 2209-2222; DOI: 10.1021/acs.inorgchem.9b02833.
- A.N. Usoltsev, S.A. Adonin, A.S. Novikov, P.A. Abramov, M.N. Sokolov, V.P. Fedin, Chlorotellurate(IV) supramolecular associates with “trapped” Br2: features of non-covalent halogen•••halogen interactions in crystalline phases, CrystEngComm, (2020) In press. DOI: 10.1039/C9CE01820D.
- S.A. Adonin, A.N. Usoltsev, A.S. Novikov, B.A. Kolesov, V.P. Fedin, M.N. Sokolov, One- and two-dimensional iodine-rich iodobismuthate(III) complexes: structure, optical properties and features of halogen bonding in solid state, Inorg. Chem., (2020) In press. DOI: 10.1021/acs.inorgchem.9b03734.
- V.N. Mikhaylov, V.N. Sorokoumov, A.S. Novikov, M.V. Melnik, A.G. Tskhovrebov, I.A. Balova, Intramolecular hydrogen bonding stabilizes trans-configuration in a mixed carbene/isocyanide PdII complexes, J. Organomet. Chem., (2020) In press. DOI: 10.1016/j.jorganchem.2020.121174.
- A. V. Buldakov, M. A. Kinzhalov, M. A. Kryukova, D. M. Ivanov, A. S. Novikov, A. S. Smirnov, G. L. Starova, N. A. Bokach and V. Y. Kukushkin, Isomorphous Series of PdII-Containing Halogen Bond Donors Exhibiting Cl/Br/I Triple Halogen Isostructural Exchange. Cryst. Growth Des. (2020) In press. DOI: 10.1021/acs.cgd.9b01631.
- O. Zaitceva, V. Bénéteau, D. S. Ryabukhin, I. I. Eliseev, M. A. Kinzhalov, B. Louis, A. V. Vasilyev and P. Pale, Cyclization of aryl 3-aryl propynoates into 4-arylcoumarins catalyzed by cyclometalated Platinum(II) complexes. Tetrahedron (2020), 131029. DOI: 10.1016/j.tet.2020.131029.
- A.N. Usoltsev, A.S. Novikov, B.A. Kolesov, K.V. Chernova, P.E. Plyusnin, V.P. Fedin, M.N. Sokolov, S.A. Adonin, Halogen•••halogen contacts in triiodide salts of pyridinium-derived cations: theoretical and spectroscopic studies, J. Mol. Struct., (2020) In press. DOI: 10.1016/j.molstruc.2020.127949.
- I.N. Klyukin, A.S. Novikov, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, Theoretical study of monocarbonyl derivatives of closo-borate anions [BnHn–1CO]– (n = 6, 10, 12): bonding and reactivity analysis, Mendeleev Commun., 30 (2020) 88–90; DOI: 10.1016/j.mencom.2020.01.029.
- M.M. Efremova, A.P. Molchanov, A.S. Novikov, G.L. Starova, A.A. Muryleva, A.V. Slita, V.V. Zarubaev, 1,3-Dipolar cycloaddition of N-allyl substituted polycyclic derivatives of isoindole-1,3-dione with nitrones and nitrile oxides: An experimental and theoretical investigation, Tetrahedron, (2020) In press. DOI: 10.1016/j.tet.2020.131104.
- A. Pulyalina, I. Faykov, V. Nesterova, M. Goikhman, I. Podeshvo, N. Loretsyan, A. Novikov, I. Gofman, A. Toikka, G. Polotskaya, Novel polyester amide membranes containing biquinoline units and complex with Cu(I): synthesis, characterization, and approbation for n-heptane isolation from organic mixtures, Polymers, 12 (2020) 645; DOI: 10.3390/polym12030645.
- T. V. Serebryanskaya, M. A. Kinzhalov, V. Bakulev, G. Alekseev, A. Andreeva, P. V. Gushchin, A. Protas, A. Smirnov, T. L. Panikorovskii, P. Lippmann, I. Ott, C. M. Verbilo, A. Zuraev, A. S. Bunev, V. Boyarskiy and N. A. Kasyanenko, Water Soluble Palladium(II) and Platinum(II) Acyclic Diaminocarbene Complexes: Solution Behavior, DNA Binding, and Antiproliferative Activity, New J. Chem., (2020), in press, DOI 10.1039/D1030NJ00060D.
- A.S. Ostras’, D.M. Ivanov, A.S. Novikov, P.M. Tolstoy, Phosphine oxides as spectroscopic halogen bond descriptors: IR and NMR correlations with interatomic distances and complexation energy, Molecules, 25 (2020) 1406; DOI: 10.3390/molecules25061406.
- N. S. Soldatova, V. V. Suslonov, T. Yu. Kissler, D. M. Ivanov, A. S. Novikov, M. S. Yusubov, P. S. Postnikov, V. Yu. Kukushkin, Halogen bonding provides heterooctameric supramolecular aggregation of diaryliodonium thiocyanate, Crystals, 10 (2020) 230; doi:10.3390/cryst10030230.
- E. A. Katlenok, M. Haukka, O. V. Levin, A. Frontera, V. Yu. Kukushkin, Supramolecular assembly of metal complexes by (Aryl)I···dz2[PtII] halogen bond, Chem. Eur. J., 26 (2020) 7692–7701; doi: 10.1002/chem.202001196.
- L. E. Zelenkov, M. L. Kuznetsov, E. V. Andrusenko, M. S. Avdontceva, G. L. Starova, N. A. Bokach, V. Yu. Kukushkin, Nickel(II)-mediated cyanamide–pyrazole coupling highlights distinct reactivity of NCNR2 and NCR nitrile ligands, New J. Chem., 44 (2020) 6979–6991, doi: 10.1039/D0NJ00704H.
- S.N. Yunusova, A.S. Novikov, O.V. Khoroshilov, I.E. Kolesnikov, M.Ya. Demakova, D.S. Bolotin, Solid-state fluorescent 1,2,4-triazole zinc(II) complexes: self-organization via bifurcated (N–H)2•••Cl contacts, Inorg. Chim. Acta, (2020) In press. DOI: 10.1016/j.ica.2020.119660.
- S.A. Adonin, M.A. Bondarenko, A.S. Novikov, M.N. Sokolov, Halogen bonding in isostructural Co(II) complexes with 2-halopyridines, Crystals, 10 (2020) 289; DOI: 10.3390/cryst10040289.
- A. Paul, L.M.D.R.S. Martins, A. Karmakar, M.L. Kuznetsov, A.S. Novikov, M.F.C. Guedes da Silva, A.J.L. Pombeiro, Environmentally benign benzyl alcohol oxidation and C-C coupling catalysed by amide functionalized 3D Co(II) and Zn(II) metal organic frameworks, J. Catal., 385 (2020) 324; DOI: 10.1016/j.jcat.2020.03.035.
- O. V. Mikolaichuk, A.V. Protas, E.A. Popova, A.S. Mikherdov, I.V. Kornyakov, R.E. Trifonov, Novel tris(5‐aryl‐1H‐tetrazol‐1‐yl)methanes and 2‐dichloromethyl‐5‐aryl‐2H‐tetrazoles and noncovalent interactions in their crystal structure, J. Heterocyclic. Chem., (2020), In press; DOI: 10.1002/jhet.3969.
- D.M. Ivanov, S.V. Baykov, A.S. Novikov, G. Romanenko, N.A. Bokach, R.A. Evarestov, V.Yu. Kukushkin, Noncovalent sulfoxide–nitrile coupling involving four center heteroleptic dipole–dipole interactions between the sulfinyl and nitrile groups, Cryst. Growth Des. 20 (2020) 3417–3428; doi: 10.1021/acs.cgd.0c00196.
- D.S. Bolotin, M.V. Il’in, V.V. Suslonov, A.S. Novikov, Symmetrical noncovalent interactions Br•••Br observed in crystal structure of exotic primary peroxide, Symmetry, 12 (2020) 637; DOI: 10.3390/sym12040637.
- M. V. Dobrynin, V. Yu. Kukushkin, R. M. Islamova, Cellulose-based hybrid glycosilicones via grafted-to metal-catalyzed hydrosilylation: “when opposites unite”, Carbohydrate Polymers, 241 (2020) 116327; doi: 10.1016/j.carbpol.2020.116327.
- I. V. Ananyev, N. A. Bokach, V. Yu. Kukushkin, Structure-directing sulfur···metal noncovalent semicoordination bonding, Acta Crystallogr., Section B, B76 (2020) 436–449; doi: 10.1107/S2052520620005685.
- A. Yu. Dubovtsev, N. V. Shcherbakov, D. V. Dar’in, V. Yu. Kukushkin, The Dichotomy of gold-catalyzed interplay between cyanamides and ynamides: controllable switch from [2+2+2] to [4+2] cycloaddition, Adv. Synth. & Catal., 362 (2020) in press; doi: 10.1002/adsc.202000434.
- D. A. Shabalin, A. Yu. Dubovtsev, E. Yu. Schmidt, B. A. Trofimov, Calcium carbide as acetylene source in cascade assemblies of hydroxypyrrolines and 3H-pyrroles from ketoximes, ChemistrySelect, 5 (2020) 3434–3437; DOI: 10.1002/slct.202000392.
- V. V. Matveevskaya, D. I. Pavlov, T. S. Sukhikh, A. L. Gushchin, A. Yu. Ivanov, T. B. Tennikova, V. V. Sharoyko, S. V. Baykov, E. Benassi, A. S. Potapov, Arene−ruthenium(II) complexes containing 11H‑indeno[1,2‑b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime: synthesis, characterization, cytotoxicity, and catalytic transfer hydrogenation of aryl ketones, ACS Omega, 2020, in press; DOI: 10.1021/acsomega.0c01204.
- M.A. Kryukova, A.V. Sapegin, A.S. Novikov, M. Krasavin, D.M. Ivanov, New crystal forms for biologically active compounds. Part 2: anastrozole as N-substituted 1,2,4-triazole in halogen bonding and lp-π interactions with 1,4-diiodotetrafluorobenzene, Crystals, 10 (2020) 371; DOI: 10.3390/cryst10050371.
- V. V. Suslonov, A. A. Eliseeva, A. S. Novikov, D. M. Ivanov, A. Yu. Dubovtsev, N. A. Bokach, V. Yu. Kukushkin, Tetrachloroplatinate(II) anion as a square-planar tecton for crystal engineering involving halogen bonding, CrystEngComm, 22 (2020) 4180-4189; doi: 10.1039/D0CE00576B.
- A. S. Mikherdov, A. S. Novikov, V. P. Boyarskiy, V. Yu. Kukushkin, The halogen bond with isocyano carbon reduces isocyanide odor, Nature Commun., 11 (2020) Article number 2921; doi: 10.1038/s41467-020-16748-x.
- A. V. Rozhkov, I. V. Ananyev, R. M. Gomila, A. Frontera, V. Yu. Kukushkin, π-Hole···dz2[PtII] interactions with electron-deficient arenes enhance phosphorescence of PtII-based luminophores, Inorg. Chem., 59 (2020) 9308–9314; doi: 10.1021/acs.inorgchem.0c01170.
- A.S. Novikov, Why [2 + 2]-cycloaddition reactions between isocyanates and imines do not occur in Pd-activation conditions?, Inorg. Chim. Acta, (2020) In press. DOI: 10.1016/j.ica.2020.119758.
- E. E. Galenko, M. A. Kryukova, M. S. Novikov, A. F. Khlebnikov, J. Org. Chem., 85 (2020) 6109–6122; doi: 10.1021/acs.joc.0c00611.
- A. N. Gusev, E. V. Braga, M. A. Kryukova, N. V. Lubomirskii, E. A. Zamnius, V. F. Shulgin, Photoluminescence of the Coordination Zinc Compounds with 3-Methyl-4-Formyl-1-Phenylpyrazol-5-one Acylhydrazones, Russ. J. Coord. Chem., 46 (2020) 251–259; doi: 10.1134/S107032842004003X.
- V. A. Ivoplgina, M. S. Chernov’yants, L. D. Popov, V. V. Suslonov, N. A. Avtushenko, N. V. Luanguzov, Structural study and thermal behavior of novel interaction product of 4-amino-5-(furan-2-yl)-4H-1,2,4-triazole-3-thione with molecular iodine, Phosphorous, Sulfur, and Silicon and the Related Elements, 195 (2020), 421-428; doi: 10.1080/10426507.2019.1700414.
- N. Toikka, A. S. Mikherdov, D. M. Ivanov, T. J. Mooibroek, N. A. Bokach, V. Yu. Kukushkin, Cyanamides as π-hole donor components of structure-directing (сyanamide)···arene noncovalent interactions, Crystal Growth & Design, 20 (2020); doi: 10.1021/acs.cgd.0c00561.
- A. V. Rozhkov, A. A. Eliseeva, S. V. Baykov, B. Galmés, A. Frontera, V. Yu. Kukushkin, Building blocks for crystal engineering involving halogen bonding: one-pot route to X-perfluoroarenes (X = Br, I) based on FeIII-assisted C–F functionalization, Crystal Growth & Design, 20 (2020); doi: 10.1021/acs.cgd.0c00606.
- I.N. Klyukin, A.S. Novikov, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, Theoretical study of closo-borate derivatives of general type [BnHn-1COR]2– (n = 6, 10, 12; R = H, CH3, NH2, OH, OCH3) – borylated analogue of organic carbonyl compounds, Polyhedron, (2020) In press. DOI: 10.1016/j.poly.2020.114682.
- A.S. Novikov, Non-covalent interactions in coordination and organometallic chemistry, Crystals, 10 (2020) 537; DOI: 10.3390/cryst10060537.
- N.S. Soldatova, P. S. Postnikov, V. V. Suslonov, T. Yu. Kissler, D. M. Ivanov, M. S. Yusubov, B. Galmés, A. Frontera, V. Yu. Kukushkin, Diaryliodonium as a double σ-hole donor: the dichotomy of thiocyanate halogen bonding provides divergent solid state arylation by diaryliodonium cations, Org. Chem. Front., 7 (2020), 2230-2242; doi: 10.1039/D0QO00678E.
- A.N. Usoltsev, S.A. Adonin, B.A. Kolesov, A.S. Novikov, V.P. Fedin, M.N. Sokolov, Opening the third century of polyhalide chemistry: thermally stable complex with “trapped” dichlorine, Chem. Eur. J., (2020) In press. DOI: 10.1002/chem.202002014.
- T. K. Nguyen, G. D. Titov, O. V. Khoroshilova, M. A. Kinzhalov, N. V. Rostovskii, Light-induced one-pot synthesis ofpyrimidine derivatives from vinyl azides. Org. Biomol. Chem. (2020) Advance Article. DOI: 10.1039/D0OB00693A.
- A.N. Usoltsev, N.A. Korobeynikov, A.S. Novikov, V.R. Shayapov, I.V. Korolkov, D.G. Samsonenko, V.P. Fedin, M.N. Sokolov, S.A. Adonin, One-dimensional supramolecular hybrid iodobismuthate(1-EtPy)3{[Bi2I9](I2)0.75}: structural features and theoretical studies of I•••I non-covalent interactions, J. Clust. Sci., (2020) In press. DOI: 10.1007/s10876-020-01843-2.
- A.M. Afanasenko, T.G. Chulkova, I.A. Boyarskaya, R.M. Islamova, A.A. Legin, B.K. Keppler, S.I. Selivanov, A.N. Vereshchagin, M.N. Elinson, M. Haukka, C,N-Chelated diaminocarbene platinum(II) complexes derived from 3,4-diaryl-1H-pyrrol-2,5-diimines and cis-dichlorobis(isonitrile)platinum(II): synthesis, cytotoxicity, and catalytic activity in hydrosilylation reactions, J. Organomet. Chem., (2020) in press; DOI: 10.1016/j.jorganchem.2020.121435.
- E. Miliutina, O. Guselnikova, N. S. Soldatova, P. Bainova, R. Elashnikov, P. Fitl, T. Kurten, M. S. Yusubov, V. Švorčík, R. R. Valiev, M. M. Chehimi, O. Lyutakov, P. S. Postnikov, Can plasmon change reaction path? Decomposition of unsymmetrical iodonium salts as an organic probe, J. Phys. Chem. Lett., 11 (2020) 5770–5776. DOI: 10.1021/acs.jpclett.0c01350.
- A.N. Usoltsev, N.A. Korobeynikov, A.S. Novikov, P.E. Plyusnin, V.P. Fedin, M.N. Sokolov, S.A. Adonin, Hybrid chlorobismuthate(III) “trapping” Br2 unit: crystal structure and theoretical investigation of non-covalent Cl···Br interactions in (1-MePy)3{[Bi2Cl9](Br2)}, Inorg. Chim. Acta, (2020) In press. DOI: 10.1016/j.ica.2020.119932.
- V.V. Sharutin, O.K. Sharutina, A.S. Novikov, S.A. Adonin, Substituent-dependent reactivity of triarylantimony(III) toward I2: isolation of [Ar3SbI]+ salt, New J. Chem., (2020) In press. DOI: 10.1039/D0NJ02774J.
- S.V. Baykov, V.P. Boyarskiy, Metal-free functionalization of azine N-oxides with electrophilic reagents, Chem Heterocycl Compd., 56 (2020) 814–823; DOI: 10.1039/d0sc02970j.
- S. Baykov, A. Semenov, M. Tarasenko, V.P. Boyarskiy, Application of amidoximes for the heterocycles synthesis, Tetrahedron Lett., (2020) In press.; DOI: 10.1016/j.tetlet.2020.152403.
- L. E. Zelenkov, D. M. Ivanov, E. K. Sadykov, N. A. Bokach, B. Galmés, A. Frontera, V. Yu. Kukushkin, Semicoordination bond breaking and halogen bond making change the supramolecular architecture of metal-containing aggregates, Crystal Growth & Design, 20 (2020); doi: 10.1021/acs.cgd.0c00999.
- S. N. Yunusova, D. S. Bolotin, M. A. Vovk, P. M. Tolstoy, V. Yu. Kukushkin, Tetrabromomethane as an organic catalyst: a kinetic study of CBr4-catalyzed Schiff condensation, Eur. J. Org. Chem., 2020, accepted. doi: 10.1002/ejoc.202001180.
- A. G. Tskhovrebov, A. S. Novikov, A. S. Kritchenkov, V. N. Khrustalev, M. Haukka, Attractive halogen···halogen interactions in crystal structure of trans-dibromogold(III) complex, Z. Kristallogr. Cryst. Mater., 235 (2020) 477–480; DOI: 10.1515/zkri-2020-0045.
- A. N. Usoltsev, A. S. Novikov, M. N. Sokolov, S. A. Adonin, Neutral heteroleptic complexes of bis(2-halopyridine)dihalocopper(II) family: how the nature of halogen atom affects supramolecular motifs and energies of halogen bonding in solid state?, Solid State Sci., (2020) In press.; DOI: 10.1016/j.solidstatesciences.2020.106441.
- V. G. Nenajdenko, N. G. Shikhaliyev, A. M. Maharramov, K. N. Bagirova, G. T. Suleymanova, A. S. Novikov, V. N. Khrustalev, A. G. Tskhovrebov, Halogenated diazabutadiene dyes: synthesis, structures, supramolecular features, and theoretical studies, Molecules, 25 (2020) 5013; DOI: 10.3390/molecules25215013.
- A. N. Usoltsev, N. A. Korobeynikov, A. S. Novikov, P. E. Plyusnin, B. A. Kolesov, V. P. Fedin, M. N. Sokolov, S. A. Adonin, One-dimensional diiodine–iodobismuthate(III) hybrids cat3{[Bi2I9](I2)3}: syntheses, stability, and optical properties, Inorg. Chem., (2020) In press.; DOI: 10.1021/acs.inorgchem.0c02599.
- A. S. Novikov, Computational insights into industrial chemistry, Computation, 8 (2020) 97; DOI: 10.3390/computation8040097.
- I. S. Dmitriev, V. Yu. Kukushkin, “Sublime generalization: discovery of the Periodic law”, a book chapter in Celebration of The Periodic Table of The Elements at The Academy of Sciences of Lisbon. A Chemistry Symposium, Coordinated by A.J.L. Pombeiro, Academia das Ciências de Lisboa Edition, 2020, pp. 8–24; e-book, ISBN 978-972-623-394-7.
- V. V. Shilovskikh, A. A. Timralieva, P. V. Nesterov, A. S. Novikov, P. A. Sitnikov, E. A. Konstantinova, A. I. Kokorin, E. V. Skorb, Melamine–barbiturate supramolecular assembly as a pH‐dependent organic radical trap material, Chem. Eur. J., (2020) In press.; DOI: 10.1002/chem.202002947.
- A. S. Novikov, Symmetry in quantum and computational chemistry, Symmetry, 12 (2020) 2028; DOI: 10.3390/sym12122028.
2019
- M.V. Il\'in, A.S. Novikov, D.S. Bolotin, Aminonitrone–iminohydroxamic acid tautomerism: theoretical and spectroscopic study, J. Mol. Struct., 1176 (2019) 759-765; DOI: 10.1016/j.molstruc.2018.09.020.
- A.A. Melekhova, A.S. Novikov, A.Yu. Dubovtsev, A.A. Zolotarev, N.A. Bokach, Tris(3,5-dimethylpyrazolyl)methane copper(I) complexes featuring one disubstituted cyanamide ligand, Inorg. Chim. Acta, 484 (2019) 69-74; DOI: 10.1016/j.ica.2018.09.024.
- I.D. Gorokh, S.A. Adonin, A.S. Novikov, M.N. Sokolov, D.G. Samsonenko, V.P. Fedin, Polybromides of pyridinium and quinolinium-type cations: cation-induced structural diversity and theoretical analysis of Br•••Br interactions, J. Mol. Struct., 1179 (2019) 725-731; DOI: 10.1016/j.molstruc.2018.11.070.
- A. Shetnev, A. Osipyan, S. Baykov, A. Sapegin, Zh. Chirkova, M. Korsakov, A. Petzer, I. Engelbrecht, J. P. Petzer, Bioorg. Med. Chem. Lett., 29 (2019), 40-46; DOI: 10.1016/j.bmcl.2018.11.018.
- A. S. Smirnov, Luisa M. D. R. S. Martins, D. N. Nikolaev, R. A. Manzhoz, V. V. Gurzhiy, A. G. Krivenko, K. O. Nikolaenko, A. V. Belyakov, A. V. Garabadzhiu, P. B. Davidovich, Structure and catalytic properties of novel copper isatin Schiff base complexes, New. J. Chem. 2019; DOI: 10.1039/C8NJ02718H.
- U. E. Dabranskaya, D. M. Ivanov, A. S. Novikov, Y. V. Matveychuk, N. A. Bokach, V. Yu. Kukushkin, Metal-involving bifurcated halogen bonding C–Br•••η2(Cl–Pt), Crystal Growth & Design, 19 (2019) 1364–1376; DOI: 10.1021/acs.cgd.8b01757.
- S.A. Adonin, M.A. Bondarenko, A.S. Novikov, P.A. Abramov, P.E. Plyusnin, M.N. Sokolov, V.P. Fedin, Halogen bonding-assisted assembly of bromoantimonate(V) and polybromide-bromoantimonate-based frameworks, CrystEngComm, (2019); In press.; DOI: 10.1039/C8CE01944D.
- S.A. Adonin, I.D. Gorokh, D.G. Samsonenko, A.S. Novikov, I.V. Korolkov, P.E. Plyusnin, M.N. Sokolov, V.P. Fedin, Binuclear and polymeric bromobismuthate complexes: crystal structures and thermal stability, Polyhedron, (2019) In press.; DOI: 10.1016/j.poly.2018.12.017.
- E. Reutskaya, A. Osipyan, A. Sapegin, A.S. Novikov, M. Krasavin, Re-thinking hydrolytic imidazoline ring expansion: a common approach to the preparation of medium-sized rings via side chain insertion into [1.4]oxa- and [1.4]thiazepinone scaffold, J. Org. Chem., (2019) In press.; DOI: 10.1021/acs.joc.8b02805.
- A. A. Eliseeva, D. M. Ivanov, A. S. Novikov, V. Yu. Kukushkin, Recognition of p-hole donor ability of iodopentafluorobenzene – a conventional s-hole donor for crystal engineering involving halogen bonding, CrystEngComm, 21 (2019) 616–628; doi: 10.1039/C8CE01851K.
- D. V. Boyarskaya, E. Bulatov, I. A. Boyarskaya, T. G. Chulkova, V. A. Rassadin, E. G. Tolstopjatova, I. E. Kolesnikov, M. S. Avdontceva, T. L. Panikorovskii, V. V. Suslonov, M. Haukka, Syntheses and structures of a series of acyclic diaminocarbene palladium(II) complexes derived from 3,4-diaryl-1 H-pyrrol-2,5-diimines and bisisocyanide palladium(II) complexes, Organomet., 38, 2 (2019) 300-309; DOI: 10.1021/acs.organomet.8b00725.
- K. T. Mahmudov, V. Yu. Kukushkin, A. V. Gurbanov, M. A. Kinzhalov, V. P. Boyarskiy, M. F. C. Guedes da Silva, A. J. L. Pombeiro, Isocyanide metal complexes in catalysis, Coord. Chem. Rev., 384 (2019) 65–89; doi: 10.1016/j.ccr.2019.01.002.
- A. V. Rozhkov, M. A. Krykova, D. M. Ivanov, A. S. Novikov, A. A. Sinelshchikova, M. V. Volostnykh, M. A. Konovalov, M. S. Grigoriev, Y. G. Gorbunova, V. Yu. Kukushkin, Reverse arene sandwich structures based upon π-hole•••[MII] (d8M = Pt, Pd) interactions, where positively charged metal centers play the role of a nucleophile, Angew. Chem. Int. Ed., 58 (2019) in press; doi: 10.1002/anie.201814062.
- A. Yu. Dubovtsev, D. V. Dar’in, M. Krasavin, V. Yu. Kukushkin, Gold-catalyzed oxidation of internal alkynes into benzils and its application for one-pot synthesis of five-, six-, and seven-membered azaheterocycles, Eur. J. Org. Chem., 2019, 1856–1864; doi: 10.1002/ejoc.201900108.
- M. A. Kryukova, A. V. Sapegin, A. S. Novikov, M. Krasavin, D. M. Ivanov, New crystal forms for biologically active compounds. Part 1: Noncovalent interactions in adducts of nevirapine with XB donors, Crystals, 9 (2019) 71; DOI: 10.3390/cryst9020071.
- A. G. Tskhovrebov, A. S. Novikov, O. V. Odintsova, V. N. Mikhaylov, V. N. Sorokoumov, T. V. Serebryanskaya, G. L. Starova, Supramolecular polymers derived from the PtII and PdII Schiff base complexes via C(sp2)–H•••Hal hydrogen bonding: combined experimental and theoretical study, J. Organomet. Chem., (2019) In press.; DOI: 10.1016/j.jorganchem.2019.01.023.
- D. S. Bolotin, V. Korzhikov-Vlakh, E. Sinitsyna, S. N. Yunusova, V. V. Suslonov, A. Shetnev, A. Osipyan, M. Krasavin, V. Yu. Kukushkin, Biocompatible zinc(II) 8-(dihydroimidazolyl)quinoline complex and its catalytic application for synthesis of poly(L,L-lactide), J. Catal., 372 (2019) 362–369; doi: 10.1016/j.jcat.2019.03.002.
- N. I. Guranova, D. Dar\'in, G. Kantin, A. S. Novikov, O. Bakulina, M. Krasavin, Rh(II)-catalyzed spirocyclization of α-diazo homophthalimides with cyclic ethers, J. Org. Chem., (2019) In press.; DOI: 10.1021/acs.joc.9b00245.
- S. A. Adonin, I. D. Gorokh, A. S. Novikov, A. N. Usoltsev, M. N. Sokolov, V. P. Fedin, Tetranuclear anionic bromobismuthate [Bi4Br18]6–: new structural type in halometalate collection, Inorg. Chem. Commun., (2019) In press.; DOI: 10.1016/j.inoche.2019.03.014.
- M.V. Dobrynin, C. Pretorius, V.K. Dumisani, A. Roodt, V.P. Boyarskiy, R.M. Islamova, Rhodium(I)-catalysed cross-linking of polysiloxanes conducted at room temperature, Journal of Catalysis, 372 (2019) 193–200; DOI: 10.1016/j.jcat.2019.03.004.
- M.A. Kinzhalov, S.V. Baykov, A.S. Novikov, M. Haukka, V.P. Boyarskiy, Intermolecular hydrogen bonding H•••Cl in crystal structure of palladium(II)-bis(diaminocarbene)complex, Z. Kristallogr. Cryst. Mater., 234 (2019) 155–164. DOI: 10.1515/zkri-2018-2100.
- I.S. Giba, V.V. Mulloyarova, G.S. Denisov, P.M. Tolstoy, “Influence of Hydrogen Bonds in 1:1 Complexes of Phosphinic Acids with Substituted Pyridines on 1H and 31P NMR Chemical Shifts”, J. Phys. Chem. A 123 (2019) 2252-2260; doi: 10.1021/acs.jpca.9b00625.
- I.D. Gorokh, S.A. Adonin, A.S. Novikov, A.N. Usoltsev, P.E. Plyusnin, I.V. Korolkov, M.N. Sokolov, V.P. Fedin, Halobismuthates with 3-iodopyridinium cations: halogen bonding-assisted crystal packing, Polyhedron, (2019) Accepted.
- A. Yu. Dubovtsev, D. V. Dar’in, V. Yu. Kukushkin, Three component [2+2+1] gold(I)-catalyzed oxidative generation of fully substituted 1,3-oxazoles involving internal alkynes, Adv. Synth. & Catal., 2019, accepted.
- S. A. Katkova, A. S. Mikherdov, M. A. Kinzhalov, A. S. Novikov, A. A. Zolotarev, V. P. Boyarskiy, V. Yu. Kukushkin, (Isocyano Group π-Hole)···[dz2-MII] Interactions at (Isocyanide)[MII] Complexes, where Positively Charged Metal Centers (d8M = Pt, Pd) Act as Nucleophiles, Chem. Eur. J., 25 (2019), 8590; DOI: 10.1002/chem.201901187.
- A. Shetnev, S. Baykov, S. Kalinin, A. Belova, V. Sharoyko, A. Rozhkov, L. Zelenkov, M. Tarasenko, E. Sadykov, M. Korsakov, M. Krasavin, 1,2,4-Oxadiazole/2-imidazoline hybrids: multi-target-directed compounds for the treatment of infectious diseases and cancer. Int. J. Mol. Sci. 20 (2019), 1699; DOI: 10.3390/ijms20071699.
- S.A. Adonin, A.S. Novikov, M.N. Sokolov, Polymeric lead(II) iodoacetate: Pb···I and I···I non‐covalent interactions in solid state, Eur. J. Inorg. Chem., (2019) In press.; DOI: 10.1002/ejic.201900349.
- S.A. Adonin, M.D. Petrov, A.S. Novikov, R.R. Shiriyazdanov, M.N. Sokolov, V.P. Fedin, 2-Chlorobenzoate complex of Cu(II): unexpected appearance of halogen•••halogen contacts in solid state, J. Clust. Sci., (2019) In press.; DOI: 10.1007/s10876-019-01574-z.
- K.V. Deriabin, E.K. Lobanovskaia, A.S. Novikov, R.M. Islamova, Platinum-catalyzed reactions between Si-H groups as a new method for cross-linking of silicones, Org. Biomol. Chem., (2019) In press.; DOI: 10.1039/C9OB00791A.
- N.I. Guranova, D. Dar’in, G. Kantin, A.S. Novikov, O. Bakulina, M. Krasavin, Fused vs. spiro: kinetic, not thermodynamic preference may direct the reaction of α-carbonyl oxonium ylides, Tetrahedron Lett., (2019) In press.; DOI: 10.1016/j.tetlet.2019.05.020.
- A. Yu. Dubovtsev, D. V. Dar’in, V. Yu. Kukushkin, Gold(I)-catalyzed oxidation of acyl acetylenes to vicinal tricarbonyls, Org. Lett., 2019, accepted.
- S.A. Adonin, A.S. Novikov, M.N. Sokolov, V.P. Fedin, Heteroleptic Cu(II) iodoacetate complex: appearance of halogen bonding in solid state, Inorg. Chem. Commun., (2019) In press.; DOI: 10.1016/j.inoche.2019.05.018.
- A.N. Usoltsev, S.A. Adonin, A.S. Novikov, M.N. Sokolov, V.P. Fedin, Halogen bonding-assisted formation of one-dimensional polybromide-bromotellurate (2-ClPyH)2{[TeBr6](Br2)}, J. Coord. Chem., (2019) In press.; DOI: 10.1080/00958972.2019.1625040.
- A. Yu. Dubovtsev, D. M. Ivanov, U. Dabranskaya, N. A. Bokach, V. Yu. Kukushkin, Saccharin guanidination via facile three-component \"two saccharins–one dialkylcyanamide\" integration, New J. Chem., 2019, in press; doi: 10.1039/C9NJ02656H.
- M. A. Kryukova, D. M. Ivanov, M. A. Kinzhalov, A. S. Novikov, A. S. Smirnov, N. A. Bokach, V. Yu. Kukushkin, Four-center nodes: supramolecular synthons based on cyclic halogen bonding, Chem. Eur. J., 25 (2019) 13671–13675; doi: 10.1002/chem.201902264.
- N. A. Shcherbina, I. V. Kazakov, N. Y. Gugin, A. S. Lisovenko, A. V. Pomogaeva, Y. V. Kondrat’ev, V. V. Suslonov, A. Y. Timoshkin, Thermal decomposition of B(C6F5)3·Py complex, Russ. J. Gen. Chem., 89, 6 (2019) 1162-1168; DOI: 10.1134/S1070363219060100.
- M. V. Il\'in, D. S. Bolotin, A. S. Novikov, I. E. Kolesnikov, V. V. Suslonov, Platinum(II)-mediated aminonitrone–isocyanide interplay: a new route to acyclic diaminocarbene complexes, Inorg. Chim. Acta, 490 (2019) 267-271; DOI: 10.1016/j.ica.2019.03.026.
- M. Ya. Demakova, R. M. Islamova, V. V. Suslonov, Palladium-catalyzed synthesis of 4-aminoquinazolines from amide oxime ethers and 2-iodobenzonitrile, Russ. J. Gen. Chem., 89, 4 (2019) 668-672; DOI: 10.1134/S1070363219040054.
- V. A. Ivolgina, M. S. Chernov\'yants, L. D. Popov, V. V. Suslonov, G. S. Borodkin, N. V. Luanguzov, N. A. Avtushenko, Perspective anti-thyroid drug 2-thioxo-5-(3,4,5-trimethoxybenzylidene) thiazolidin-4-one: X-ray and thermogravimetric characterization of two novel molecular adducts, obtained by interaction with I 2, J. Mol. Struct., 1180 (2019) 629-635; DOI: 10.1016/j.molstruc.2018.12.043.
- R. R. Kostikov, T. A. Kornilova, A. F. Khlebnikov, G. V. Shustov, A. Y. Ivanov, V. V. Suslonov, N. V. Shesternin, M. A. Kuznetsov, Spatial structure and nontrivial stereodynamics of tricyclic perhydro-1,2,4,5-tetrazines, Chem. Heterocycl. Compd., 55, 2 (2019) 172-177; DOI: 10.1007/s10593-019-02435-3.
- M. A. Kinzhalov, A. A. Eremina, A. S. Smirnov, V. V. Suslonov, V. Yu. Kukushkin, K. V. Luzyanin, Cleavage of acyclic diaminocarbene ligands at an iridium(III) center. Recognition of a new reactivity mode for carbene ligands, Dalton Trans., 48, 22 (2019) 7571-7582; DOI: 10.1039/c9dt01138b.
- D.P. Zimin, D.V. Dar’in, A.A. Eliseeva, A.S. Novikov, V.A. Rassadin, V.Yu. Kukushkin, Gold-catalyzed functionalization of semicarbazides with terminal alkynes to achieve substituted semicarbazones, Eur. J. Org. Chem., (2019) In press.; DOI: 10.1002/ejoc.201901108.
- S.A. Adonin, M.A. Petrov, P.A. Abramov, A.S. Novikov, M.N. Sokolov, V.P. Fedin, Halogen bonding in heteroleptic Cu(II) 2-iodobenzoates, Polyhedron, (2019) In press.; DOI: 10.1016/j.poly.2019.07.020.
- A. Gusev, E. Braga, E. Zamnius, M. Kiskin, M. Kryukova, A. Baryshnikova, B. Minaev, G. Baryshnikov, H. Agren, W. Linert, Structure and excitation-dependent emission of novel zinc complexes with pyridyltriazoles. RCS Adv., 9 (2019) 22143-22152; DOI: 10.1039/c9ra02491c.
- S. Baykov, M. Tarasenko, L.E. Zelenkov, S. Kasatkina, P. Savko, A. Shetnev, Diastereoselective Opening of Bridged Anhydrides by Amidoximes Providing Access to 1,2,4-Oxadiazole/Norborna(e)ne Hybrids, Eur. J. Org. Chem., (2019) In press.; DOI: 10.1002/ejoc.201900843.
- E.A. Kostenko, S.V. Baykov, A.S. Novikov, V.P. Boyarskiy, Nucleophilic properties of the positively charged metal center in the solid state structure of Palladium(II)-Terpyridine complex, J. Mol. Struct., (2019) In press.; DOI: 10.1016/j.molstruc.2019.126957.
- I.D. Gorokh, S.A. Adonin, A.N. Usoltsev, A.S. Novikov, D.G. Samsonenko, S.V. Zakharov, M.N. Sokolov, V.P. Fedin, Bromide complexes of bismuth with 4-bromobenzyl-substituted cations of pyridinium family, J. Mol. Struct., (2019) In press.; DOI: 10.1016/j.molstruc.2019.126955.
- P.S. Teterina, M.M. Efremova, E.V. Sirotkina, A.S. Novikov, O.V. Khoroshilova, A.P. Molchanov, A highly efficient and stereoselective cycloaddition of nitrones to N-arylitaconimides, Tetrahedron Lett., (2019) In press.; DOI: 10.1016/j.tetlet.2019.151063.
- V. A. Ilichev, Silantyeva L. I., Grishin I. D., Rozhkov A. V., Rumyantcev R. V., Fukin G. K., Bochkarev M. N., Cerium(III) complexes with azolyl-substituted thiophenolate ligands: synthesis, structure and red luminescence, RSC Adv., 9 (2019), 24110-24116; DOI: 10.1039/C9RA03199E.
- A. M. Afanasenko, A. S. Novikov, T. G. Chulkova, Y. M. Grigoriev, I. E. Kolesnikov, S. I. Selivanov, G. L. Starova, A. A. Zolotarev, A. N. Vereshchagin, M. N. Elinson, Intermolecular interactions-photophysical properties relationships in phenanthrene-9,10-dicarbonitrile assemblies, J. Mol. Struct. (2019) in press; DOI: 10.1016/j.molstruc.2019.07.036.
- M. A. Kinzhalov, K. V. Luzyanin, Coord. Chem. Rev., 399 (2019), 213014; DOI: 10.1016/j.ccr.2019.213014.
- K. Geyl, S. Baykov, M. Tarasenko, L.E. Zelenkov, V. Matveevskaya, V.P. Boyarskiy, Convenient entry to N-pyridinylureas with pharmaceutically privileged oxadiazole substituents via the acid-catalyzed C–H activation of N-oxides, Tetrahedron Lett., (2019) In press.; DOI: 10.1016/j.tetlet.2019.151108.
- S.A. Adonin, M.A. Bondarenko, A.S. Novikov, P.A. Abramov, P.E. Plyusnin, M.N. Sokolov, V.P. Fedin, Antimony(V) bromide and polybromide complexes with N-alkylated quinolinium or isoquinolinium cations: substituent-dependent assembly of polymeric frameworks, Z. Anorg. Allg. Chem., (2019) In press.; DOI: 10.1002/zaac.201900165.
- M. V. Kashina, M. A. Kinzhalov, ;A. S. Smirnov, D. M. Ivanov, A. S. Novikov, V. Yu. Kukushkin, Dihalomethanes as Bent Bifunctional XB/XB-Donating Building Blocks for Construction of Metal-involving Halogen Bonded Hexagons, Chemistry — An Asian Journal, DOI: 10.1002/asia.201901127, in press.
- S.A. Adonin, M.A. Bondarenko, A.S. Novikov, P.A. Abramov, M.N. Sokolov, V.P. Fedin, Halogen bonding in the structures of pentaiodobenzoic acid and its salts, CrystEngComm, (2019) In press.; DOI: 10.1039/C9CE01106D.
- A. M. Afanasenko, A. S. Novikov, T. G. Chulkova, Y. M. Grigoriev, I. E. Kolesnikov, S. I. Selivanov, G. L. Starova, A. A. Zolotarev, A. N. Vereshchagin, M. N. Elinson, Structural Data of Phenanthrene-9,10-dicarbonitriles, Data in Brief (2019) in press; DOI: 10.1016/j.dib.2019.104605.
- M. V. Il’in, A. A. Sysoeva, D. S. Bolotin, A. S. Novikov, V. V. Suslonov, E. V. Rogacheva, L. A. Kraeva, V. Yu. Kukushkin, Aminonitrones as Highly Reactive Bifunctional Synthons. Expedient One-pot Route to 5-Amino-1,2,4-triazoles and 5-Amino-1,2,4-oxadiazoles – Potential Antimicrobials Targeting Multi-drug Resistant Bacteria, New J. Chem. (2019) in press. DOI: 10.1039/C9NJ04529E.
- A. Guseva, V. Shul\'gin, E. Braga, E. Zamnius, N. Lyubomirskiy, M. Kryukova, W. Linert, Strong enhancement of ionic luminescence Dy(III), Tb(III), Eu(III) and Sm (III) by pyridyltriazoles. J. Lumin., 212 (2019) 315-321; DOI: 10.1016/j.jlumin.2019.04.055.
- A.N. Kornev, Y.S. Panova, V.V. Sushev, D.F. Dorado Daza, A.S. Novikov, A.V. Cherkasov, G.K. Fukin, G.A. Abakumov, The nature of P(σ2λ3↔σ2λ1) dualism: 3a,6a-diaza-1,4-diphosphapentalene as a form of stabilized singlet phosphinidene, Inorg. Chem., (2019) In press.; DOI: 10.1021/acs.inorgchem.9b02690.
- S.A. Adonin, M.A. Bondarenko, A.S. Novikov, P.E. Plyusnin, I.V. Korolkov, M.N. Sokolov, V.P. Fedin, Five new Sb(V) bromide complexes and their polybromide derivatives with pyridinium-type cations: structures, thermal stability and features of halogen•••halogen contacts in solid state, Inorg. Chim. Acta, (2019) In press.; DOI: 10.1016/j.ica.2019.119278.
- A. V. Rozhkov, D. M. Ivanov, A. S. Novikov, I. V. Ananyev, N. A. Bokach, V. Yu. Kukushkin, Metal-involving halogen bond Ar–I∙∙∙[dz2PtII] in the platinum acetylacetonate complex, CrystEngComm, 2019, in press; doi: 10.1039/C9CE01568J.
- A. V. Rozhkov, S. N. Eliseeva, S. Baykov, L. E. Zelenkov, D. Goryachii, I. V. Taydakov, Copper(I) ionic complexes based on imidazo[4,5-f][1,10]phenanthrolin diimine chelating ligands: crystal structures, photo- and electroluminescent properties, New J. Chem., (2019), in press; DOI: 10.1039/C9NJ05109K.
- A. S. Mikherdov, S. V. Baikov, I. K. Proskurina, A. A. Shetnev, A. D. Kotov, Synthesis and properties of C,N-chelated carbene complexes of palladium(II) with 2-aminobenzo[d]thiazole fragment, Russ. J. Gen. Chem., 89 (2019) 2062-2068; DOI: 10.1134/S1070363219100128.
- A. A. Eliseeva, D. M. Ivanov, A. S. Novikov, A. V. Rozhkov, I. V. Kornyakov, A. Yu. Dubovtsev, V. Yu. Kukushkin, Hexaiododiplatinate(II) as a useful supramolecular synthon for halogen bond involving crystal engineering, Dalton Trans., 2019, in press; doi: 10.1039/C9DT04221K.
- S.A. Adonin, A.S. Novikov, K.V. Chernova, D.A. Vinnik, S.V. Taskaev, I.V. Korolkov, E.V. Ilyina, A.A. Pavlov, V.V. Novikov, M.N. Sokolov, V.P. Fedin, Heteroleptic copper(II) complexes with 2-bromo-5-methylpyridine: structures, features of non-covalent interactions and magnetic behavior, Inorg. Chim. Acta, (2019) In press. DOI: 10.1016/j.ica.2019.119333
- I.N. Klyukin, A.S. Novikov, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, QTAIM analysis of mono-hydroxy derivatives of closo-borate anions [BnHn–1OH]2– (n = 6, 10, 12), Russ. J. Inorg. Chem. 64 (2019) 1-4; DOI: 10.1134/S0036023619140031.
- S. Presnukhina, M. Tarasenko, S. Baykov, S. N. Smirnov, V. P. Boyarskiy, A. Shetnev, M. K. Korsakov, Entry into (E)-3-(1,2,4-oxadiazol-5-yl)acrylic acids via a one-pot ring-opening/ring-closing/retro-Diels-Alder reaction sequence, Tetrahedron Lett., 2019, in press; DOI: 10.1016/j.tetlet.2019.151543.
2018
- A. S. Novikov, Theoretical confirmation of existence of X•••Au non-covalent contacts, Inorg. Chim. Acta, 471 (2018) 126-129, DOI: 10.1016/j.ica.2017.11.009.
- E. A. Daines, D. S. Bolotin, N. A. Bokach, V. V. Gurzhiy, A. P. Zhdanov, K. Yu. Zhizhin, N. T. Kuznetsov, Push-pull alkenes bearing closo-decaborate cluster generated via nucleophilic addition of carbanions to borylated nitrilium salts, Inorg. Chim. Acta, 471 (2018) 372.
- R. S. Chay, B. G. M. Rocha, A. J. L. Pombeiro, V. Yu. Kukushkin, K. V. Luzyanin, Platinum complexes with chelating acyclic aminocarbene ligands work as catalysts for the hydrosilylation of alkynes, ACS Omega, 3 (2018) 683–871; doi: 10.1021/acsomega.7b01688.
- V.A. Rozentsvet, O.A. Stotskaya, V.P. Ivanova, M.G. Kuznetsova, P.M. Tolstoy, S.V. Kostjuk, Structural characterization of polybutadiene synthesized via cationic mechanism, J. Polym. Sci. A 56 (2018) 387–398; DOI: 10.1002/pola.28905.
- M. Promzeleva, T.V. Volkova, A.N. Proshin, O.I. Siluykov, A. Mazur, P.M. Tolstoy, S.P. Ivanov, F. Kamilov, I.V. Terekhova, Improved biopharmaceutical properties of oral formulations of 1,2,4-thiadiazole derivative with cyclodextrins: in vitro and in vivo evaluation, ACS Biomater. Sci. Eng. 4 (2018) 491–501; DOI: 10.1021/acsbiomaterials.7b00887.
- M. Shevtsov, B. Nikolaev, Y. Marchenko, L. Yakovleva, N. V. Skvorzov, A. Mazur, P. Tolstoy, V. Ryzhov, G. Multhoff, Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs), Int. J. Nanomedicine 13 (2018) 1471–1482; DOI: 10.2147/IJN.S152461.
- E.Yu. Tupikina, M. Bodensteiner, P.M. Tolstoy, G.S. Denisov, I.G. Shenderovich, P=O Moiety as an Ambidextrous Hydrogen Bond Acceptor, J. Phys. Chem. C 122 (2018) 1711–1720; DOI: 10.1021/acs.jpcc.7b11299.
- S. A. Adonin, I. D. Gorokh, A. S. Novikov, D. G. Samsonenko, P. E. Plyusnin, M. N. Sokolov, V. P. Fedin, Bromine-rich complexes of bismuth: experimental and theoretical studies, Dalton Trans., 47 (2018) 2683–2689; DOI: 10.1039/C7DT04779G.
- V.V. Mulloyarova, I.S. Giba, M.A. Kostin, G.S. Denisov, I.G. Shenderovich, P.M. Tolstoy, Cyclic Trimers of Phosphinic Acids in Polar Aprotic Solvent: Symmetry, Chirality and H/D Isotope Effects on NMR Chemical Shifts, Phys. Chem. Chem. Phys. 20 (2018) 4901–4910; DOI: 10.1039/C7CP08130H.
- A. S. Mikherdov, A. S. Novikov, M. A. Kinzhalov, V. P. Boyarskiy, G. L. Starova, V. Yu. Kukushkin, Halides held by bifurcated chalcogen–hydrogen bonds. Effect of μ(S,N–H)Cl contacts on dimerization of Cl(Carbene)PdII species, Inorg. Chem., 57 (2018) 3420–3433; DOI: 10.1021/acs.inorgchem.8b00190.
- A. S. Mikherdov, A. S. Novikov, M. A. Kinzhalov, A. A. Zolotarev, V. P. Boyarskiy, Intra-/intermolecular bifurcated chalcogen bonding in crystal structure of thiazole/thiadiazole derived binuclear (diaminocarbene)PdII complexes, Crystals, 8 (2018) 112; DOI:10.3390/cryst8030112.
- E. Yu. Tupikina, G. S. Denisov, S. M. Melikova, S. Yu. Kucherov, P. M. Tolstoy, New look at the Badger-Bauer rule: correlations of spectroscopic IR and NMR parameters with hydrogen bond energy and geometry. FHF complexes, J. Mol. Struct. 1164 (2018) 129–136; DOI: 10.1016/j.molstruc.2018.03.018.
- M.A. Kinzhalov, E. A. Popova, M. L. Petrov, O. V. Khoroshilova, K. T. Mahmudov, A. J. L. Pombeiro, Pnicogen and chalcogen bonds in cyclometalated iridium(III) complexes, Inorg. Chim. Acta, 477 (2018) 31–33 DOI: 10.1016/j.ica.2018.02.029.
- S.A. Adonin, M.A. Bondarenko, P.A. Abramov, A.S. Novikov, P.E. Plyusnin, M.N. Sokolov, V.P. Fedin, Bromo- and polybromoantimonates (V): structural and theoretical studies of hybrid halogen-rich halometalate frameworks, Chem. Eur. J., 40 (2018) 10165-10170; DOI: 10.1002/chem.201801338.
- M. Bulatova, A.A. Melekhova, A.S. Novikov, D.M. Ivanov, N.A. Bokach, Redox reactive (RNC)CuII species stabilized in the solid state via halogen bond with I2, Z. Kristallogr. Cryst. Mater., 233 (2018) 371–377; DOI: 10.1515/zkri-2017-2107.
- V.K. Burianova, D.S. Bolotin, A.S. Mikherdov, A.S. Novikov, P.P. Mokolokolo, A. Roodt, V.P. Boyarskiy, D. Dar'in, M. Krasavin, V.V. Suslonov, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, Mechanism of generation of closo-decaborato amidrazones. Intramolecular non-covalent B–H•••π(Ph) interaction determines stabilization of the configuration around the amidrazone C=N bond, New J. Chem., 42 (2018) 8693–8703; DOI: 10.1039/c8nj01018h.
- V.A. Dmitriev, M.M. Efremova, A.S. Novikov, V.V. Zarubaev, A.V. Slita, A.V. Galochkina, G.L. Starova, A.V. Ivanov, A.P. Molchanov, Highly efficient and stereoselective cycloaddition of nitrones to indolyl- and pyrrolylacrylates, Tetrahedron Lett., 59 (2018) 2327–2331; DOI: 10.1016/j.tetlet.2018.04.066.
- M. V. Il’in, D. S. Bolotin, V. V. Suslonov, V. Yu. Kukushkin, Facile selective synthesis of 2-methyl-5-amino-1,2,4-oxadiazolium bromides as further targets for nucleophilic additions (Letter), New J. Chem., 42 (2018) 9373–9376; doi: 10.1039/C8NJ01682H.
- A. S. Mikherdov, M. A. Kinzhalov, A. S. Novikov, V. P. Boyarskiy, I. A. Boyarskaya, M. S. Avdontceva, V. Yu. Kukushkin, Ligation-enhanced π-hole•••π interactions involving isocyanides. Effect of π-hole•••π non-covalent bonding on conformational stabilization of acyclic diaminocarbene ligands, Inorg. Chem., 57 (2018) 6722–6733; doi: 10.1021/acs.inorgchem.8b01027.
- A. V. Rozhkov, A. S. Novikov, D. M. Ivanov, D. S. Bolotin, N. A. Bokach, V. Yu. Kukushkin, Structure-directing weak interactions with 1,4-diiodotetrafluorobenzene convert 1D-arrays of [MII(acac)2] species into 3D-networks, Crystal Growth & Design, 18 (2018) 3626–3636; doi: 10.1021/acs.cgd.8b00408.
- A.S. Novikov, D.S. Bolotin, Tautomerism of amidoximes and other oxime species, J. Phys. Org. Chem., 31 (2018) e3772; DOI: 10.1002/poc.3772.
- M.M. Efremova, A.S. Novikov, R.R. Kostikov, T.L. Panikorovsky, A.V. Ivanov, A.P. Molchanov, Regio- and diastereoselectivity of the cycloaddition of nitrones with N-propadienylindole and pyrroles, Tetrahedron, 74 (2018) 174–183; DOI: 10.1016/j.tet.2017.11.056.
- S.A. Adonin, I.D. Gorokh, A.S. Novikov, D.G. Samsonenko, I.V. Korolkov, M.N. Sokolov, V.P. Fedin, Bromobismuthates: cation-induced structural diversity and Hirshfeld surface analysis of cation-anion contacts, Polyhedron, 139 (2018) 282-288; DOI: 10.1016/j.poly.2017.11.002.
- M.A. Kinzhalov, S.A. Katkova, E.P. Doronina, A.S. Novikov, I.I. Eliseev, V.A. Ilichev, A.A. Kukinov, G.L. Starova, N.A. Bokach, Red photo- and electroluminescent half-lantern cyclometalated dinuclear platinum(II) complex, Z. Kristallogr. Cryst. Mater., 233 (2018) 795–802; DOI: 10.1515/zkri-2018-2075.
- A.N. Usoltsev, S.A. Adonin, P.A. Abramov, A.S. Novikov, V.R. Shayapov, P.E. Plyusnin, I.V. Korolkov, M.N. Sokolov, V.P. Fedin, 1D and 2D polybromotellurates(IV): structural studies and thermal stability, Eur. J. Inorg. Chem., 2018 (2018) 3264-3269; DOI: 10.1002/ejic.201800383.
- S. N. Yunusova, D. S. Bolotin, V. V. Suslonov, M. A. Vovk, P. M. Tolstoy, V. Yu. Kukushkin, 3-Dialkylamino-1,2,4-triazoles via ZnII-catalyzed acyl hydrazide–dialkylcyanamide coupling, ACS Omega, 3 (2018) 7224–7234. DOI: 10.1021/acsomega.8b01047.
- K. Sotiriadis, P. Macova, A. S. Mazur, P. M. Tolstoy, A. Viani, A solid state NMR and in-situ infrared spectroscopy study on the setting reaction of magnesium sodium phosphate cement, J. Non-Cryst. Solids, 498 (2018) 49–59; doi: 10.1016/j.jnoncrysol.2018.06.006.
- V.K. Burianova, A.S. Mikherdov, D.S. Bolotin, A.S. Novikov, P.P. Mokolokolo, A. Roodt, V.P. Boyarskiy, V.V. Suslonov, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, Electrophilicity of Aliphatic Nitrilium closo-Decaborate Clusters: Hyperconjugation Provides an Unexpected Inverse Reactivity Order, J. Organomet. Chem., 870 (2018) 97–103. DOI: 10.1016/j.jorganchem.2018.06.017.
- T. Sharonova, V. Pankrat’eva, P. Savko, S. Baykov, A. Shetnev, Facile Room-Temperature Assembly of the 1,2,4-Oxadiazole Core from Readily Available Amidoximes and Carboxylic Acids. Tetrahedron Lett. 59 (2018) 2824-2827; DOI: 10.1016/j.tetlet.2018.06.019.
- S.A. Adonin, L.I. Udalova, P.A. Abramov, A.S. Novikov, I.V. Yushina, I.V. Korolkov, E.Yu. Semitut, T.A. Derzhavskaya, K.J. Stevenson, P.A. Troshin, M.N. Sokolov, V.P. Fedin, A novel family of polyiodo-bromoantimonate(III) complexes: cation-driven self-assembly of photoconductive metal-polyhalide frameworks, Chem. Eur. J., 24 (2018), 14707–14711; DOI: 10.1002/chem.201802100.
- V.K. Burianova, D.S. Bolotin, A.S. Novikov, I.E. Kolesnikov, V.V. Suslonov, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, Nucleophilic addition of hydrazine and benzophenone hydrazone to 2-acetonitrilium closo-decaborate cluster: Structural and photophysical study, Inorg. Chim. Acta, 482 (2018) 838-845; DOI: 10.1016/j.ica.2018.07.038.
- D. P. Zimin, D. V. Dar’in, V. A. Rassadin, V. Yu. Kukushkin, Gold-catalyzed hydrohydrazidation of terminal alkynes, Org. Lett., 20 (2018) 4880–4884; doi: 10.1021/acs.orglett.8b02019.
- M. A. Kinzhalov, M. V. Kashina, A. S. Mikherdov, E. A. Mozheeva, A. S. Novikov, A. S. Smirnov, D. M. Ivanov, M. A. Kryukova, A. Yu. Ivanov, S. N. Smirnov, V. Yu. Kukushkin, K. V. Luzyanin, Dramatically enhanced solubility of halide-containing organometallic species in diiodomethane: the role of solvent•••complex halogen bonding, Angew. Chem. Int. Ed., 57 (2018) 12785–12789; doi: 10.1002/anie.201807642.
- A.S. Novikov, Strong metallophilic interactions in nickel coordination compounds, Inorg. Chim. Acta, 483 (2018) 21-25; DOI: 10.1016/j.ica.2018.08.002.
- A. Osipyan, A. Sapegin, A.S. Novikov, M. Krasavin, Rare medium-sized rings prepared via hydrolytic imidazoline ring expansion (HIRE), J. Org. Chem., 83 (2018) 9707–9717; DOI: 10.1021/acs.joc.8b01210.
- J. C. Gee, B. A. Fuller, H.-M. Lockett, G. Sedghi, C. M. Robertson, K. V. Luzyanin, Visible light accelerated hydrosilylation of alkynes using platinum–[acyclic diaminocarbene] photocatalysts, Chem. Comm., 54 (2018) 9450–9453; doi: 10.1039/c8cc04287j.
- L. E. Zelenkov, D. M. Ivanov, M. S. Avdontceva, A. S. Novikov, N. A. Bokach, Tetrachloromethane as halogen bond donor toward metal-bound halides, Z. Kristallogr., (2018), in press. DOI: 10.1515/zkri-2018-2111.
- S.V. Baykov, U. Dabranskaya, D.M. Ivanov, A.S. Novikov, V.P. Boyarskiy, Pt/Pd and I/Br isostructural exchange provides formation of C–I•••Pd, C–Br•••Pt, and C–Br•••Pd metal-involving halogen bonding, Cryst. Growth Des., 18 (2018) 5973–5980; DOI: 10.1021/acs.cgd.8b00762.
- Y.S. Panova, A.V. Sheyanova, N.V. Zolotareva, V.V. Sushev, A.V. Arapova, A.S. Novikov, E.V. Baranov, G.K. Fukin, A.N. Kornev, 2,2′-Azobispyridine in phosphorus coordination chemistry: a new approach to 1,2,4,3-triazaphosphole derivatives, Eur. J. Inorg. Chem., 2018 (2018) 4245–4254; DOI: 10.1002/ejic.201800831.
- M. A. Kryukova, A. V. Sapegin, A. S. Novikov, M. Krasavin, D. M. Ivanov, Non-covalent interactions observed in nevirapinium pentaiodide hydrate which include the rare I4–I−···O=C halogen bonding, Z. Kristallogr., (2018) In press. DOI: 10.1515/zkri-2018-2081.
- M.A. Kinzhalov, S.V. Baykov, A.S. Novikov, M. Haukka, V.P. Boyarskiy, Intermolecular hydrogen bonding H•••Cl in crystal structure of palladium(II)-bis(diaminocarbene)complex, Z. Kristallogr. Cryst. Mater., (2018), In press. DOI: 10.1515/zkri-2018-2100.
- 10.1134/S1070428018080213.
- S.A. Adonin, I.D. Gorokh, A.S. Novikov, D.G. Samsonenko, I.V. Yushina, M.N. Sokolov, V.P. Fedin, Halobismuthates with halopyridinium cations: appearance or non-appearance of unusual colouring, CrystEngComm, 20 (2018) 7766-7772; DOI: 10.1039/C8CE01749B.
- I.D. Gorokh, S.A. Adonin, P.A. Abramov, A.S. Novikov, M.N. Sokolov, V.P. Fedin, New structural type in polybromide-bromometalate hybrids: (Me3NH)3{[Bi2Br9](Br2)} – crystal structure and theoretical studies of non-covalent Br•••Br interactions, Inorg. Chem. Commun., 98 (2018) 169-173; DOI: 10.1016/j.inoche.2018.10.027.
- V. P. Boyarskii, M. V. Sangaranarayanan, I. A. Boyarskaya, E. G. Tolstopyatova, T. G. Chulkova, Electrochemical Reduction of Trichlorobiphenyls: Mechanism and Regioselectivity, Russ. J. Gen. Chem., 2018, Vol. 88, No. 10, 2058–2066; DOI: 10.1134/S1070363218100055.
- G. Casella, M. Casarin, V. Yu. Kukushkin, M. L. Kuznetsov, The reaction between indazole and Pd-bound isocyanides – a theoretical mechanistic study, Molecules, 23 (2018) 2942; doi:10.3390/molecules23112942.
- A. S. Novikov, D. M. Ivanov, Z. M. Bikbaeva, N. A. Bokach, V. Yu. Kukushkin, Noncovalent interactions involving iodofluorobenzenes: the interplay of halogen bonding and weak lp(O)•••p-holearene interactions, Crystal Growth & Design, 18 (2018) 7641–7654; doi: 10.1021/acs.cgd.8b01457.
- M. A. Kinzhalov, S. N. Parfenova, A. S. Novikov, E. A. Katlenok, M. V. Puzyk, M. S. Avdontceva, N. A. Bokach, Cyclometalated iridium(III) complexes featuring disubstituted cyanamides, ChemistrySelect, 3 (2018) 11875–11880; DOI: 10.1002/slct.201802900.
2017
- M. L. Kuznetsov, V. Yu. Kukushkin. Diversity of reactivity modes upon interplay between Au(III)-bound isocyanides and cyclic nitrones: a theoretical consideration. Dalton Trans., 46 (2017) 786–802, DOI: 10.1039/C6DT03840A.
- B. Koeppe, S.A. Pylaeva, C. Allolio, D. Sebastiani, E.T.J. Nibbering, G.S. Denisov, H.-H. Limbach, P.M. Tolstoy. Polar solvent fluctuations drive proton transfer in hydrogen bonded complexes of carboxylic acid with pyridines: NMR, IR and ab initio MD study, Phys. Chem. Chem. Phys., 19 (2017) 1010–1028, DOI: 10.1039/C6CP06677A.
- D. M. Ivanov, M. A. Kinzhalov, A. S. Novikov, I. V. Ananyev, A. A. Romanova, V. P. Boyarskiy, M. Haukka, V. Yu. Kukushkin. The H2C(X)–X•••X– (X = Cl, Br) halogen bonding of dihalomethanes, Cryst. Growth Des., 17 (2017) 1353–1362, DOI: 10.1021/acs.cgd.6b01754.
- D.S. Bolotin, M.V. Il'in, I.E. Kolesnikov, V.V. Suslonov, Y. Novozhilov, O. Ronzhina, M. Krasavin, V.P. Boyarskiy, R. Koen, A. Roodt. Fluorescent (pyrazolyl acetoxime)ZnII complexes: Synthetic, structural, and photophysical studies. Inorg. Chim. Acta, 455 (2017) 9–14, DOI: 10.1016/j.ica.2016.10.008.
- D.S. Bolotin, V.A. Rassadin, N.A. Bokach, V.Yu. Kukushkin. Metal-involving generation of aminoheterocycles from N-substituted cyanamides: Toward sustainable chemistry (a Minireview). Inorg. Chim. Acta, 455 (2017) 446–454, DOI: 10.1016/j.ica.2016.02.025.
- E. V. Andrusenko, E. V. Kabin, A. S. Novikov, N. A. Bokach, G. L. Starova, V. Yu. Kukushkin, Metal-mediated generation of triazapentadienate-terminated di- and trinuclear μ2-pyrazolate NiII species and control of their nuclearity. New J. Chem., 41 (2017) 316–325, DOI: 10.1039/C6NJ02962K.
- M. A. Kinzhalov, A. S. Novikov, A. N. Chernyshev, V. V. Suslonov. Intermolecular hydrogen bonding H···Cl- in the solid palladium(II)-diaminocarbene complexes. Z. Krist. – Cryst. Mater., 232 (2017) 299–305, DOI:10.1515/zkri-2016-2018.
- D. S. Bolotin, M. V. Il’in, A. S. Novikov, N. A. Bokach, V. V. Suslonov, Vadim Yu. Kukushkin. Trinuclear (Aminonitrone)ZnII Complexes as Key Intermediates in Zinc(II)-mediated Generation of 1,2,4-Oxadiazoles from Amidoximes and Nitriles. New J. Chem., 41 (2017) 1940–1952, DOI: 10.1039/C6NJ03508F.
- A. S. Smirnov, D. N. Nikolaev, V. V. Gurzhiy, S. N. Smirnov, V. S. Suslonov, A. V. Garabadzhiu, P. B. Davidovich, Conformational stabilization of isatin Schiff bases – biologically active chemical probes, RSC Advances, 7 (2017) 10070–10073, DOI: 10.1039/C6RA26779C.
- M. Brusnikina, O. Silyukov, M. Chislov, T. Volkova, A. Proshin, A. Mazur, P. Tolstoy, I. Terekhova, Effect of cyclodextrin complexation on solubility of novel anti-Alzheimer 1,2,4-thiadiazole derivative. J. Therm. Anal. Calorim. 2017, 130, 443-450. DOI: 10.1007/s10973-017-6252-1.
- A. A. Melekhova, A. S. Smirnov, A. S. Novikov, T. L. Panikorovskiy, N. A. Bokach, V. Yu. Kukushkin, Copper(I)-catalyzed 1,3-dipolar cycloaddition of ketonitrones to dialkylcyanamides. A step toward sustainable generation of 2,3-dihydro-1,2,4-oxadiazoles, ACS Omega, 2 (2017) 1380–1391, DOI: 10.1021/acsomega.7b00130.
- A. S. Novikov, D. M. Ivanov, M. S. Avdontceva, V. Yu. Kukushkin, Diiodomethane as halogen bond donor toward metal-bound halides, CrystEngComm, 2017, accepted, DOI: 10.1039/C7CE00346C.
- T. B. Anisimova, M. A. Kinzhalov, M. F. C. Guedes da Silva, A. S. Novikov, V. Yu. Kukushkin, A. J. L. Pombeiro, K. V. Luzyanin, Addition of N-nucleophiles to gold(III)-bound isocyanides leading to short-lived gold(III)-ADC complexes, New J. Chem., 2017, in press, DOI: 10.1039/C7NJ00529F.
- K. V. Luzyanin, A. N. Marianov, P. G. Kislitsyn, V. P. Ananikov. Substrate-selective C–H functionalization for the preparation of organosulfur compounds from crude oil-derived components, ACS Omega, 2 (2017), 1419–142, DOI: 10.1021/acsomega.7b00137.
- A.A. Melekhova, A.S. Novikov, N.V.Rostovskii, P.A.Sakharov, T.L.Panikorovskii, N.A.Bokach, Open-chain hemiketal is stabilized by coordination to a copper(II), Inorg. Chem. Commun, 79 (2017) 82–85, DOI: 10.1016/j.inoche.2017.03.024.
- E.V.Sirotkina, M.M.Efremova, A.S.Novikov, V.V.Zarubaev, I.R.Orshanskaya, G.L.Starova, R.R.Kostikov, A.P.Molchanov, Regio- and diastereoselectivity of the cycloaddition of aldonitrones with benzylidenecyclopropane: An experimental and theoretical study, Tetrahedron, 73 (2017) 3025–3030, DOI: 10.1016/j.tet.2017.04.014.
- D.S. Bolotin, M.Ya. Demakova, A. Legin, V.V. Suslonov, A.Nazarov, M. Jakupec, B.K. Keppler, V.Yu. Kukushkin, Amidoxime Platinum(II) Complexes: pH-Dependent Highly Selective Generation and Cytotoxic Activity, New J. Chem., (2017) DOI: 10.1039/C7NJ00982H.
- N.G. Voron’ko, S.R. Derkach, M.A. Vovk, P.M. Tolstoy, «Complexation of κ-carrageenan with gelatin in the aqueous phase analyzed by 1H NMR kinetics and relaxation», Carbohydr. Polym. 2017, 169, 117-126. DOI: 10.1016/j.carbpol.2017.04.010.
- M. L. Kuznetsov, V. Yu. Kukushkin, Metal-mediated addition of N-nucleophiles to isocyanides: mechanistic aspects, Molecules, 22 (2017) 1141; doi:10.3390/molecules22071141.
- Z. M. Bikbaeva, A. S. Novikov, V. V. Suslonov, N. A. Bokach, V. Yu. Kukushkin, Metal-mediated reactions between dialkylcyanamides and acetamidoxime generate unusual (Nitrosoguanidinate)Nickel(II) complexes, Dalton Trans., 2017, accepted.
- A. S. Novikov, D. S. Bolotin, Tautomerism of amidoximes and other oxime species, J. Phys. Org. Chem. (2017) Accepted.
- M. V. Il’in, D. S. Bolotin, A. S. Novikov, V. V. Suslonov, N. V. Chezhina, M. P. Bubnov, V. K. Cherkasov, G. J. S. Venter, A. Roodt, Square-planar Aminonitronate Transition Metal Complexes(M = CuII, NiII, PdII, and PtII), Inorg. Chim. Acta (2017) Accepted.
- S.A. Pylaeva, H. Elgabarty, D. Sebastiani, P.M. Tolstoy, “Symmetry and dynamics of FHF– anion in vacuum, in CD2Cl2 and in CCl4. Ab initio MD study of fluctuating solvent-solute hydrogen and halogen bonds”, Phys. Chem. Chem. Phys. 2017, 19, 26107-26120. DOI: 10.1039/C7CP04493C.
- D. S. Bolotin, N. A. Bokach, M. Ya. Demakova, V. Yu. Kukushkin, Metal-involving synthesis and reactions of oximes, Chem. Rev., 117 (2017) accepted.
- Usoltsev A.N., Adonin S.A., Novikov A.S., Samsonenko D.G., Sokolov M.N., Fedin V.P. “One-dimensional polymeric polybromotellurates (IV): structural and theoretical insights into halogen•••halogen contacts”, CrystEngComm 2017, Accepted.
- Adonin S.A., Gorokh I.D., Novikov A.S., Abramov P.A., Sokolov M.N., Fedin V.P. “Halogen contacts-induced unusual coloring in Bi(III) bromide complex: anion-to-cation charge transfer via Br•••Br interactions”, Chem. Eur. J. 2017, Accepted.
- M. A. Kinzhalov, A. S. Legkodukh, T. B. Anisimova, A. S. Novikov, V. V. Suslonov, K. V. Luzyanin, V. Yu. Kukushkin, Tetrazol-5-ylidene gold(III) complexes from sequential [2 + 3] cycloaddition of azide to metal-bound isocyanides and N4-alkylation, Organometallics, 36 (2017) accepted.
- S. A. Katkova, M. A. Kinzhalov, P. M. Tolstoy, A. S. Novikov, V. P. Boyarskiy, A. Yu. Ananyan, P. V. Gushchin, M. Haukka, A. A. Zolotarev, A. Yu. Ivanov, S. S. Zlotsky, V. Yu. Kukushkin, Diversity of isomerization patterns and protolytic forms in aminocarbene PdII and PtII complexes formed upon addition of N,N’-diphenylguanidine to metal-activated isocyanides, Organometallics, 36 (2017) accepted.
- V.A. Rozentsvet, V.G. Kozlov, O.A. Stotskaya, N.A. Sablina, V.P. Ivanova, P.M. Tolstoy, “Kinetic parameters of cationic polymerization of 1,3-dienes”, Russian Chemical Bulletin, International Edition, 2017, 66(6), 1088-1093.
- A.A. Osipov, V.E. Eremyashev, A.S. Mazur, P.M. Tolstoi, L.M. Osipova, «Structure of Cesium–Borosilicate Glasses According to NMR Spectroscopy», Glass Phys. Chem. 2017, 43(4), 287-293. DOI: 10.1134/S1087659617040113.
- A.V. Artem’ev, I.Yu. Bagryanskaya, E.P. Doronina, P.M. Tolstoy, A.L. Gushchin, M.I. Rakhmanova, A.Yu. Ivanov, A.O. Suturina, “Facile self-assembly of new luminescent clusters containing a silver-centered tetracapped [Ag@Ag4(µ3-P)4] tetrahedron, inscribed within a N12 icosahedron”, Dalton Trans. 2017, 46, 12425-12429, DOI: 10.1039/C7DT02597a.
- Z. M. Bikbaeva, D. M. Ivanov, A. S. Novikov, I. V. Ananyev, N. A. Bokach, V. Yu. Kukushkin, Electrophilic–nucleophilic dualism of nickel(II) toward Ni•••I non-covalent interactions: semicoordination of iodine centers via electron belt and halogen bonding via s-hole, Inorg. Chem., 56 (2017), accepted.
- Kinzhalov M.A., Eremina A.A., Ivanov D.M., Novikov A.S., Katlenok E.A., Balashev K.P., Suslonov V.V. “Halogen and chalcogen bonding in dichloromethane solvate of cyclometalated iridium(III)-isocyanide complex”, Z. Kristallogr. Cryst. Mater. 2017, In press. DOI 10.1515/zkri-2017-2065.
- Melekhova A.A., Novikov A.S., Panikorovskii T.L., Bokach N.A., Kukushkin V.Yu. “Novel family of homoleptic copper(I) complexes featuring disubstituted cyanamides: combined synthetic, structural, and theoretical study”, New J. Chem. 2017, Accepted.
- Bolotin D.S., Bikbaeva Z. M., Novikov A. S., Suslonov V. V., Bokach N. A. "A Dimetallic Aminonitrone Nickel(II) Complex: Further Insights into Metal-Mediated Nucleophilic Activation of Amidoximes", ChemistrySelect, 2017, accepted.
- Adonin S.A., Gorokh I.D., Abramov P.A., Novikov A.S., Korolkov I.V., Sokolov M.N., Fedin V.P. “Chlorobismuthates trapping dibromine: formation of two-dimensional supramolecular polyhalide networks with Br2 linkers”, Eur. J. Inorg. Chem. 2017, Accepted. DOI: 10.1002/ejic.201700908.
- V.A. Rozentsvet, O.A. Stotskaya, V.P. Ivanova, M.G. Kuznetsova, P.M. Tolstoy, S.V. Kostjuk, "Structural characterization of polybutadiene synthesized via cationic mechanism", J. Polym. Sci. A 2017, accepted. DOI: 10.1002/pola.28905.
- Adonin S.A., Gorokh I.D., Novikov A.S., Samsonenko D.G., Korolkov I.V., Sokolov M.N., Fedin V.P. “Bromobismuthates: cation-induced structural diversity and Hirshfeld surface analysis of cation-anion contacts”, Polyhedron 2017, In press. DOI: 10.1016/j.poly.2017.11.002.
- R.M. Islamova, M.V. Dobrynin, A.V. Vlasov, A.A. Eremina, M.A. Kinzhalov, I.E. Kolesnikov, A.A. Zolotarev, E.A. Masloborodova, K.V. Luzyanin,"Iridium(III)-catalysed cross-linking of polysiloxanes leading to the thermally resistant luminescent silicone rubbers", Catal. Sci. Technol., 2017, accepted. DOI:10.1039/c7cy02013a.
- Efremova M.M., Novikov A.S., Kostikov R.R., Panikorovsky T.L., Ivanov A.V., Molchanov A.P. “Regio- and diastereoselectivity of the cycloaddition of nitrones with N-propadienylindole and pyrroles”, Tetrahedron 2017, Accepted. DOI: 10.1016/j.tet.2017.11.056.
- E.Yu. Tupikina, A.A. Efimova, G.S. Denisov, P.M. Tolstoy, The NMR chemical shift of a Helium atom as a probe for electronic structure of FH, F–, (FHF)– and FH2+, J. Phys. Chem. A 121 (2017) 9654–9662; DOI: 10.1021/acs.jpca.7b10189.
- I. S. Aliyarova, E. Yu. Tupikina, D. M. Ivanov, V. Yu. Kukushkin, Metal-involving halogen bonding including gold(I) as a nucleophilic partner. The case of isomorphic dichloroaurate(I)∙halomethane cocrystals, Inorg. Chem., 61 (2022); doi: 10.1021/acs.inorgchem.1c03482.
- M. Nikishina, L. Perelomov, Y. Atroshchenko, E. Ivanova, L. Mukhtorov, P. Tolstoy, Sorption of fulvic acids and their compounds with heavy metal ions on clay minerals, Soil Syst., 6 (2022), 2; DOI: 10.3390/soilsystems6010002.
- D. M. Ivanov, N. A. Bokach, V. Yu Kukushkin, A. Frontera, Metal centers as nucleophiles: oxymoron of halogen bond-involving crystal engineering, Chem. Eur. J., 28 (2022) e202103173. DOI: 10.1002/chem.202103173.
- S. A. Katkova, A. S. Mikherdov, E. V. Sokolova, A. S. Novikov, G. L. Starova, M. A. Kinzhalov, Intermolecular (Isocyano group)×××PtII Interactions Involving Coordinated Isocyanides in Cyclometalated PtII Complexes, J. Mol. Struct., 1253 (2022) 132230. DOI: 10.1016/j.molstruc.2021.132230.
- M. A. Bondarenko, P. A. Abramov, A. S. Novikov, M. N. Sokolov, S. A. Adonin, Cu(II) pentaiodobenzoate complexes: “super heavy carboxylates” featuring strong halogen bonding, Polyhedron 214 (2022) 115644; DOI: 10.1016/j.poly.2021.115644.
- A. D. Mironova, M. A. Mikhaylov, A. M. Maksimov, K. A. Brylev, A. L. Gushchin, D. V. Stass, A. S. Novikov, I. V. Eltsov, P. A. Abramov, M. N. Sokolov, Phosphorescent complexes of {Mo6I8}4+ and {W6I8}4+ with perfluorinated aryl thiolates featuring unusual molecular structures, Eur. J. Inorg. Chem. (2022) In press.; DOI: 10.1002/ejic.202100890.
- N. F. Romashev, P. A. Abramov, I. V. Bakaev, I. S. Fomenko, D. G. Samsonenko, A. S. Novikov, K. K. H. Tong, D. Ahn, P. V. Dorovatovskii, Y. V. Zubavichus, A. A. Ryadun, O. A. Patutina, M. N. Sokolov, M. V. Babak, A. L. Gushchin, Heteroleptic Pd(II) and Pt(II) complexes with redox-active ligands: synthesis, structure, and multimodal anticancer mechanism, Inorg. Chem. (2022) In press.; DOI: 10.1021/acs.inorgchem.1c03314.
- V. Rozentsvet, N. Sablina, D. Ulyanova, S. Kostjuk, P. Tolstoy, Structure of terminal units of polybutadiene synthesized via anionic mechanism, Polym. Bull. 79 (2022), 1239-1256; DOI: 10.1007/s00289-021-03549-5.
- M. A. Kinzhalov, D. M. Ivanov, A. A. Melekhova, N. A. Bokach, A. Frontera, V. Yu. Kukushkin, Chameleonic metal-bound isocyanides: a π-donating CuI-center imparts nucleophilicity to the isocyanide carbon toward halogen bonding, Inorg. Chem. Front., 9 (2022) 1655–1665; doi: 10.1039/D2QI00034b.
- M.V. ll'in, A.A. Sysoeva, A.S. Novikov, D.S. Bolotin, Diaryliodoniums as Hybrid Hydrogen- and Halogen-Bond-Donating Organocatalysts for the Groebke–Blackburn–Bienaymé Reaction. J. Org. Chem., 87 (2022) 4569–4579; DOI:10.1021/acs.joc.1c02885.
- A. S. Novikov, Theoretical investigation on non-covalent interactions, Crystals 12 (2022) 167; DOI: 10.3390/cryst12020167.
- M. V. Grudova, A. S. Kubasov, V. N. Khrustalev, A. S. Novikov, A. S. Kritchenkov, V. G. Nenajdenko, A. V. Borisov, A. G. Tskhovrebov, Exploring supramolecular assembly space of cationic 1,2,4-selenodiazoles: effect of the substituent at the carbon atom and anions, Molecules 27 (2022) 1029; DOI: 10.3390/molecules27031029.
- A. S. Novikov, Non-covalent interactions in organic, organometallic, and inorganic supramolecular systems relevant for medicine, materials science, and catalysis, Crystals 12 (2022) 246; DOI: 10.3390/cryst12020246.
- V. A. Dodonov, O. A. Kushnerova, R. V. Rumyantsev, A. S. Novikov, V. K. Osmanov, I. L. Fedushkin, Cycloaddition of isoselenocyanates to sodium and magnesium metallacycles, Dalton Trans. (2022) In press.; DOI: 10.1039/D1DT04366H.
- V. V. Suslonov, N. S. Soldatova, P. S. Postnikov, G. Resnati, V. Yu. Kukushkin, D. M. Ivanov, N. A. Bokach, Diaryliodonium tetracyanometallates self-assemble into halogen bonded square-like arrays, Crystal Growth & Design, 22 (2022); doi: 10.1021/acs.cgd.2c00175.
- K. Sotiriadis, K. Aspiotis, A. Mazur, P. Tolstoy, E. Badogiannis, S. Tsivilis, Characterization of old concrete from a heritage structure of Inousses cluster of islands, Lect. Notes Civ. Eng., 209 (2022) 80–89; DOI: 10.1007/978-3-030-90788-4_7.
- F. Shakirova, U. Markova, P. Tolstoy, A. Bulatov, A. Shishov, A new hydrophobic deep eutectic solvent based on thymol and 4-(dimethylamino)benzaldehyde: derivatization and microextraction of urea, J. Mol. Liquids, 353 (2022) 118820; DOI: 10.1016/j.molliq.2022.118820.
- A. Mazur, P. Tolstoy, K. Sotiriadis, 13С, 27Al and 29Si NMR investigation of the hydration kinetics of Portland-limestone cement pastes containing CH3-COO–R+ (R=H or Na), Materials, 15 (2022) 2004; DOI: 10.3390/ma15062004.
- M. A. Kostin, S. A. Pylaeva, P. M. Tolstoy, Phosphine oxides as NMR and IR spectroscopic probes for the estimation of the geometry and energy of hydrogen bonds: PO…H-A hydrogen bonds, Phys. Chem. Chem. Phys., 24 (2022) 7121–7133; DOI: 10.1039/D1CP05939D.
- V. A. Rozentsvet, D. M. Ulyanova, N. A. Sablina, S. V. Kostjuk, P. M. Tolstoy, I. A. Novakov, Cationic polymerization of butadiene using alkyl aluminum compounds as co-initiators: an efficient approach toward solid polybutadienes, Polym. Chem., 3 (2022) 1596–1607; DOI: 10.1039/d1py01684a.
- N. S. Soldatova, P. S. Postnikov, D. M. Ivanov, O. V. Semyonov, O. S. Kukurina, O. Guselnikova, Y. Yamauchi, T. Wirth, V. V. Zhdankin, M. S. Yusubov, R. M. Gomila, A. Frontera, G. Resnati, V. Yu. Kukushkin, Zwitterionic iodonium species afford halogen bond-based porous organic frameworks, Chemical Science, 2022, accepted.
- E. I. Chikunova, V. Yu Kukushkin, A. Yu. Dubovtsev, Atom-economic Synthesis of β-Ketosulfones Based on Gold-catalyzed Highly Regioselective Hydration of Alkynylsulfones, Green Chem., 2022, DOI: 10.1039/D2GC00541G.
- A. A. Yakubenko, A.M. Puzyk, V. O. Korostelev, V. V. Mulloyarova, E. Yu. Tupikina, P. M. Tolstoy, A. S. Antonov, Self-association of diphenylpnictoginic acids in solution and solid state: covalent vs. hydrogen bonding, Phys. Chem. Chem. Phys., 24 (2022), 7882–7892; DOI: 10.1039/d2cp00286h.
- M. V. Dobrynin, S. O. Kasatkina, S. V. Baykov, P. Y. Savko, N. S. Antonov, A. S. Mikherdov, V. P. Boyarskiy, R. M. Islamova, Cyclometallated platinum(II) complexes for obtaining phenyl-containing silicone rubbers via catalytic hydrosilylation reaction, Russ. J. Gen. Chem., 92 (2022), 79–84; DOI: 10.1134/S107036322201011X.
- K. K. Geyl, S. V. Baykov, S. A. Kalinin, A. S. Bunev, M. A. Troshina, T. V. Sharonova, M. Y. Skripkin, S. O. Kasatkina, S. I. Presnukhina, A. A. Shetnev, M. Y. Krasavin, V. P. Boyarskiy, Synthesis, structure, and antiproliferative action of 2-pyridyl urea-based Cu(II) complexes, Biomedicines, 10 (2022), 461; DOI: 10.3390/biomedicines10020461.
- S. Kasatkina, K. Geyl, S. Baykov, M. Novikov, V. Boyarskiy, “Urea to urea” approach: access to unsymmetrical ureas bearing pyridyl substituents, Adv. Synth. Catal., 364 (2022), 1295–1304; DOI: 10.1002/adsc.202101490.
- S. V. Baykov, A. V. Semenov, S. I. Presnukhina, A. S. Novikov, A. A. Shetnev, V. P. Boyarskiy. Hydrogen vs. halogen bonding in crystals of 2,5-dibromothiophene-3-carboxylic acid derivatives. J. Mol. Struct., 1260 (2022), 132785; DOI: 10.1016/j.molstruc.2022.132785.
- S. V. Baykov, M. V. Tarasenko, A. V. Semenov, E. A. Katlenok, A. A. Shetnev, V. P. Boyarskiy. Dualism of 1,2,4-oxadiazole ring in noncovalent interactions with carboxylic group. J. Mol. Struct., 1262 (2022), 132974; DOI: 10.1016/j.molstruc.2022.132974.
- E. Zelenkov, A. A. Eliseeva, S. V. Baykov, D. M. Ivanov, A. I. Sumina, R. M. Gomila, A. Frontera, V. Yu. Kukushkin, N. A. Bokach, Inorganic–organic {dz2-MIIS4}···p-hole stacking in reverse sandwich structures. The case of cocrystals of group 10 metal dithiocarbamates with electron-deficient arenes, Inorg. Chem. Front., 9 (2022) accepted.
- I. R. Mardaleishvili, А. В. Вологжанина, А. С. Новиков, A. I. Shienok, L. S. Kol’tsova, N. L. Zaichenko, V. A. Nadtochenko, А. Г. Цховребов, Hydrogen bonding in the crystal of 1,1'-((1E,1'E)-(pyridine-3,4-diylbis(azanylylidene))bis(methanylylidene))-bis(naphthalen-2-ol) acetonitrile solvate: combined experimental and theoretical study, JSC, 63 (2022) 510-512; DOI: 10.26902/JSC_id91711.
- I. N. Klyukin, A. V. Kolbunova, A. S. Novikov, A. V. Nelyubin, N. A. Selivanov, A. Y. Bykov, A. A. Klyukina, A. P. Zhdanov, K. Y. Zhizhin, N. T. Kuznetsov, Protonation of borylated carboxonium derivative [2,6-B10H8O2CCH3]−: theoretical and experimental investigation, Int. J. Mol. Sci., 23 (2022) 4190; DOI: 10.3390/ijms23084190.
- I. S. Giba, P. M. Tolstoy, V. V. Mulloyarova, A phosphonic acid anion and acid dimer dianion stabilized by proton transfer in OHN hydrogen bonds – models of structural motifs in blend polymer membranes, Phys. Chem. Chem. Phys., 24 (2022) 11362-11369; DOI: 10.1039/D2CP00551D.
- A. Pulyalina, K. Grekov, V. Tataurova, A. Senchukova, A. Novikov, I. Faykov, G. Polotskaya, Effect of ionic liquid on formation of copolyimide ultrafiltration membranes with improved rejection of La3+, Sci. Rep., 12 (2022) 8200; DOI: 10.1038/s41598-022-12377-0.
- M. V. Grudova, A. S. Novikov, A. S. Kubasov, V. N. Khrustalev, A. A. Kirichuk, V. G. Nenajdenko, A. G. Tskhovrebov, Aurophilic interactions in cationic three-coordinate gold(I) bipyridyl/isocyanide complex, Crystals, 12 (2022) 613; DOI: 10.3390/cryst12050613.
- N. A. Korobeynikov, A. N. Usoltsev, T. S. Sukhikh, A. S. Novikov, I. V. Korolkov, V. P. Fedin, M. N. Sokolov, S. A. Adonin, Halogen-rich halorhenates(IV): (Me4N)2{[ReX6](X2)} (X = Cl, Br), Polyhedron, 221 (2022) 115876; DOI: 10.1016/j.poly.2022.115876.
- A. Pulyalina, N. Tian, A. Senchukova, I. Faykov, M. Ryabikova, A. Novikov, N. Saprykina, G. Polotskaya, Application of cyclized polyacrylonitrile for ultrafiltration membrane fouling mitigation, Membranes, 12 (2022) 489; DOI: 10.3390/membranes12050489.
- N. Y. Shmelev, T. H. Okubazghi, P. A. Abramov, M. I. Rakhmanova, A. S. Novikov, M. N. Sokolov, A. L. Gushchin, Asymmetric coordination mode of phenanthroline-like ligands in gold(I) complexes: a case of the antichelate effect, Cryst. Growth Des., (2022) In press. DOI: 10.1021/acs.cgd.2c00287.
- A. A. Artemjev, A. A. Astafiev, A. V. Vologzhanina, A. S. Kubasov, G. M. Burkin, A. S. Novikov, A. S. Kritchenkov, A. A. Kirichuk, A. G. Tskhovrebov, Triarylazoimidazole-ZnII, CdII, and HgII complexes: structures, photophysics, and antibacterial properties, Crystals, 12 (2022) 680; DOI: 10.3390/cryst12050680.
- N. A. Korobeynikov, A. N. Usoltsev, P. A. Abramov, A. S. Novikov, M. N. Sokolov, S. A. Adonin, Bromine-rich tin(IV) halide complexes: experimental and theoretical examination of Br···Br noncovalent interactions in crystalline state, Polyhedron, 222 (2022) 115912; DOI: 10.1016/j.poly.2022.115912.
- E. A. Katlenok, A. V. Rozhkov, R. R. Ramazanov, R. R. Valiev, O. V. Levin, D. O. Goryachiy, I. V. Taydakov, M. L. Kuznetsov, V. Yu. Kukushkin, Photo- and electroluminescent neutral iridium(III) complexes bearing imidoylamidinate ligands Inorg. Chem., 61 (2022) accepted.
- A.S. Novikov, D.S. Bolotin, Хеnon Derivatives as Aerogen Bond-Donating Catalysts for Organic Transformations: A Theoretical Study on the Metaphorical "Spherical Cow in a Vacuum" Provides Insights into Noncovalent Organocatalysis, J. Org. Chem., 2022, in press. DOI: 10.1021/acs.joc.2c00680.
- A. S. Novikov, Self-healing polymers, Polymers, 14 (2022) 2261; DOI: 10.3390/polym14112261.
- O. S. Taniya, V. V.Fedotov, A. S. Novikov, L. K. Sadieva, A. P. Krinochkin, I. S. Kovalev, D. S. Kopchuk, G. V. Zyryanov, Y. Liu, E. N. Ulomsky, V. L. Rusinov, V. N. Charushin, Abnormal push-pull benzo[4,5]imidazo[1,2-a][1,2,3]triazolo[4,5-e]pyrimidine fluorophores in planarized intramolecular charge transfer (PLICT) state: Synthesis, photophysical studies and theoretical calculations, Dyes and Pigments, 204 (2022) 110405; DOI: 10.1016/j.dyepig.2022.110405.
- P. A. Petrov, E. A. Filippova, T. S. Sukhikh, A. S. Novikov, M. N. Sokolov, Sterically hindered tellurium(IV) catecholate as a Lewis acid, Inorg. Chem., 61 (2022) 9184–9194; DOI: 10.1021/acs.inorgchem.2c00751.
- V. А. Rozentsvet, D. M. Ulyanova, N. А. Sablina, M. G. Kuznetsov, P. M. Tolstoy, Polymerization of penta-1,3-diene using cationic catalytic systems based on organoaluminum compounds, Russ. Chem. Bull. 71 (2022) 787-795; DOI: 10.1007/s11172-022-3479-1.
- E. Yu. Tupikina, A. A. Titova, M. V. Kaplanskiy, E. R. Chakalov, M. A. Kostin, P. M. Tolstoy, Estimations of OH···N hydrogen bond length from positions and intensities of IR bands, Spectrochim. Acta A 275 (2022), 121172; DOI: 10.1016/j.saa.2022.121172.
- V.V. Suslonov, N.S. Soldatova, D.M. Ivanov, B. Galmés, A. Frontera, G. Resnati, P.S. Postnikov, V.Y. Kukushkin, N.A. Bokach, Diaryliodonium Tetrachloroplatinates(II): Recognition of a Trifurcated Metal-Involving μ3-I···(Cl,Cl,Pt) Halogen Bond, Crystal Growth and Design, 21 (2021), 5360-5372; DOI: 10.1021/acs.cgd.1c00654.
- L.E. Zelenkov, A.A. Eliseeva, S.V. Baykov, V.V. Suslonov, B. Galmés, A. Frontera, V.Y. Kukushkin, D.M. Ivanov, N.A. Bokach, Electron belt-to-s-hole switch of noncovalently bound iodine(i) atoms in dithiocarbamate metal complexes, Inorganic Chemistry Frontiers, 8 (2021), 2505-2517; DOI: 10.1039/d1qi00314c.
- M. V. Dobrynin, I. V. Mongilev, A. A. Lezov, I. Perevyazko, P. Tolstoy, Y. A. Anufrikov, A. Yu. Shasherina, P. Vlasov, V. Yu. Kukushkin, R. M. Islamova, Block-copolymeric Maltodextrin-based Amphiphilic Glycosilicones as Surface-active Systems, New J. Chem. 46 (2022) 14849–14858. DOI: 10.1039/D2NJ02285K.
- N.A. Shcherbina, I.V. Kazakov, A.S. Lisovenko, M.A. Kryukova, I.S. Krasnova, M.Bodensteiner, A.Y. Timoshkin, Molecular complexes of non-chelating polydentate Lewis bases with group 13 Lewis acids: crystal structure and computed energy of stepwise donor–acceptor bond formation, Mendeleev Comm. 32 (2022), 74-77; DOI: 10.1016/j.mencom.2022.01.024.
- V. G. Bardakov, A.A. Yakubenko, V. A. Verkhov, A. S. Antonov, Organoboron Derivatives of 1,8-Bis(dimethylamino)naphthalene: Synthesis, Structure, Stability, and Reactivity, Organometallics, 41 (2022) 1501-1508; DOI: 10.1021/acs.organomet.2c00115.
- V. Y. Mikshiev, P. M. Tolstoy, A. M. Puzyk, S. O. Kirichenkoa, A. S. Antonov, peri-Interactions in 1,8-bis(dimethylamino)naphthalene ortho-ketimine cations facilitate [1,5]-hydride shift: selective synthesis of 1,2,3,4-tetrahydrobenzo[h]quinazolines, Org. Biomol. Chem., 20 (2022) 4559-4568; DOI: 10.1039/D2OB00674J.
- M.V. Il'in, A.S. Novikov, D.S. Bolotin, Sulfonium and Selenonium Salts as Noncovalent Organocatalysts for the Multicomponent Groebke–Blackburn–Bienaymé Reaction, J. Org. Chem., (2022) in press. DOI: 10.1021/acs.joc.2c01141.
- N. V. Shcherbakov, G. D. Titov, E. I. Chikunova, I. Filippov, N. V. Rostovskii, V. Yu. Kukushkin, A. Yu. Dubovtsev, Modular Approach to Non-aromatic and Aromatic Pyrroles through Gold-catalyzed [3+2] Cycloaddition of 2H-Azirines and Ynamides, Org. Chem. Front., (2022), DOI: 10.1039/D2QO01105K.
- E.R. Chakalov, E.Yu. Tupikina, D.M. Ivanov, E.V. Bartashevich, P.M. Tolstoy, The Distance between Minima of Electron Density and Electrostatic Potential as a Measure of Halogen Bond Strength, Molecules, 27 (2022) 4848; doi: 10.3390/molecules27154848.
- E. Yu. Tupikina, Non-covalent interactions in the glutathione peroxidase active center and their influence on the enzyme activity, Org. Biomol. Chem., 50 (2022); doi: 10.1039/d2ob00890d.
- E.Yu. Tupikina, M.V. Sigalov, P.M. Tolstoy, Simultaneous Estimation of Two Coupled Hydrogen Bond Geometries from Pairs of Entangled NMR Parameters: The Test Case of 4-Hydroxypyridine Anion, Molecules, 27 (2022) 3923; doi: 10.3390/molecules27123923.
- V. G. Nenajdenko, N. G. Shikhaliyev, A. M. Maharramov, G. T. Atakishiyeva, A. A. Niyazova, N. A. Mammadova, A. S. Novikov, I. V. Buslov, V. N. Khrustalev, A. G. Tskhovrebov, Structural organization of dibromodiazadienes in the crystal and identification of Br···O halogen bonding involving the nitro group, Molecules 27 (2022) 5110; DOI: 10.3390/molecules27165110.
- A. A. Muravev, A. S. Ovsyannikov, G. V. Konorov, D. R. Islamov, K. S. Usachev, A. S. Novikov, S. E. Solovieva, I. S. Antipin, Thermodynamic vs. kinetic control in synthesis of O-donor 2,5-substituted furan and 3,5-substituted pyrazole from heteropropargyl precursor, Molecules 27 (2022) 5178; DOI: 10.3390/molecules27165178.
- N. A. Korobeynikov, A. N. Usoltsev, A. S. Novikov, P. A. Abramov, M. N. Sokolov, S. A. Adonin, Selenium(IV) polybromide complexes: structural diversity driven by halogen and chalcogen bonding, Molecules 27 (2022) 5355; DOI: 10.3390/molecules27165355.
- Y. A. Mezenov, S. Bruyere, A. Krasilin, E. Khrapova, S. V. Bachinin, P. V. Alekseevskiy, S. Shipiloskikh, P. Boulet, S. Hupont, A. Nomine, B. Vigolo, A. S. Novikov, T. Belmonte, V. A. Milichko, Insights into solid-to-solid transformation of MOF amorphous phases, Inorg. Chem. (2022) In press. DOI: 10.1021/acs.inorgchem.2c01978.
- N. Kulachenkov, M. Barsukova, P. Alekseevskiy, A. A. Sapianik, M. Sergeev, A. Yankin, A. A. Krasilin, S. Bachinin, S. Shipilovskikh, P. Poturaev, N. Medvedeva, E. Denislamova, P. S. Zelenovskiy, V. V. Shilovskikh, Y. Kenzhebayeva, A. Efimova, A. S. Novikov, A. Lunev, V. P. Fedin, V. A. Milichko, Dimensionality mediated highly repeatable and fast transformation of coordination polymer single crystals for all-optical data processing, Nano Lett. (2022) In press. DOI: 10.1021/acs.nanolett.2c01770.
- A. S. Mikherdov, R. A. Popov, A. S. Smirnov, A. A. Eliseeva, A. S. Novikov, V. P. Boyarskiy, R. M. Gomila, A. Frontera, V. Yu. Kukushkin, N. A. Bokach, Isocyanide and сyanide entities form isostructural halogen bond-based supramolecular networks featuring five-center tetrafurcated halogen···C/N bonding, Crystal Growth & Design, 22 (2022); doi: 10.1021/acs.cgd.2c00686.
- Y. V. Torubaev, A. V. Rozhkov, I. V. Skabitsky, R. M. Gomila, A. Frontera, V. Yu. Kukushkin, Heterovalent chalcogen bonding. Supramolecular assembly driven by the occurrence of the tellurium(II)···Ch(I) (Ch = S, Se, Te) linkage, Inorg. Chem. Front., 9 (2022); doi: 10.1039/D2QI01420C.
- A. Sapegin, E. Rogacheva, L. Kraeva, M. Gureev, M. Dogonadze, T. Vinogradova, P. Yablonsky, S. Balalaie, S. V. Baykov, M. Krasavin, Novel 5-nitrofuran-tagged imidazo-fused azines and azoles amenable by the Groebke–Blackburn–Bienaymé multicomponent reaction: activity profile against ESKAPE pathogens and mycobacteria, Biomedicines, 10 (2022), 2203; DOI: 10.3390/biomedicines10092203.
- S. Baykov, M. Tarasenko, V. Kotlyarova, A. Shetnev, L. E. Zelenkov, I. A. Boyarskaya, V. P. Boyarskiy, 2‐(1,2,4‐Oxadiazol‐5‐yl)aniline as a new scaffold for blue luminescent materials, ChemistrySelect, 7 (2022), e202201201; DOI: 10.1002/slct.202201201.
- A. Shetnev, M. Tarasenko, V. Kotlyarova, S. Baykov, K. Geyl, S. Kasatkina, N. Sibinčić, V. Sharoyko, E. V. Rogacheva, L. A. Kraeva, External oxidant-free and transition metal-free synthesis of 5-amino-1,2,4-thiadiazoles as promising antibacterials against ESKAPE pathogen strains, Mol. Divers., (2022); DOI: 10.1007/s11030-022-10445-1.
- A. S. Nebalueva, A. A. Timralieva, R. V. Sadovnichii, A. S. Novikov, M. V. Zhukov, A. S. Aglikov, A. A. Muravev, T. V. Sviridova, V. P. Boyarskiy, A. L. Kholkin, E. V. Skorb, Piezo-responsive hydrogen-bonded frameworks based on vanillin-barbiturate conjugates, Molecules, 27 (2022), 5659; DOI: 10.3390/molecules27175659.
- H. Rajasekaran, P. Jerome, E. V. Eliseenkov, V. P. Boyarskiy, N. Bhuvanesh, R. Karvembu, Half-sandwich Ru(II)-thioamide complexes as catalysts for one pot synthesis of aromatic 1,5-diketones, J. Organomet. Chem., 965–966 (2022), 122322. DOI: 10.1016/j.jorganchem.2022.122322.
- E. V. Sokolova, M. A. Kinzhalov, A. S. Smirnov, A. M. Cheranyova, D. M. Ivanov, V. Yu. Kukushkin, N. A. Bokach, Polymorph-dependent phosphorescence of cyclometalated platinum(II) complexes and its relation to non-covalent interactions, ACS Omega, 2022; doi: 10.1021/acsomega.2c04110.
- N. K. Neumolotov, N. A. Selivanov, A. Yu. Bykov, I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, K. Yu. Zhizin, N. T. Kuznetsov, New methods for preparation of the monofluorosubstituted derivative of the closo-borate anion [2-B10H9F]2–, its properties, and analysis of its reactivity, Russ. J. Inorg. Chem. 67 (2022) 1583–1590; DOI: 10.1134/S0036023622600861.
- V. K. Osmanov, E. V. Chipinsky, V. N. Khrustalev, A. S. Novikov, R. K. Askerov, A. O. Chizhov, G. N. Borisova, A. V. Borisov, M. M. Grishina, M. N. Kurasova, A. A. Kirichuk, A. S. Peregudov, A. S. Kritchenkov, A. G. Tskhovrebov, Facile access to 2-selenoxo-1,2,3,4-tetrahydro-4-quinazolinone scaffolds and corresponding diselenides via cyclization between methyl anthranilate and isoselenocyanates: synthesis and structural features, Molecules 27 (2022) 5799; DOI: 10.3390/molecules27185799.
- Y. V. Demyanov, E. H. Sadykov, M. I. Rakhmanova, A. S. Novikov, I. Y. Bagryanskaya, A. V. Artem’ev, Tris(2-pyridyl)arsine as a new platform for design of luminescent Cu(I) and Ag(I) complexes, Molecules 27 (2022) 6059; DOI: 10.3390/molecules27186059.
- M. A. Bondarenko, A. S. Novikov, M. N. Sokolov, S. A. Adonin, Heteroleptic Zn(II)–pentaiodobenzoate complexes: structures and features of halogen–halogen non-covalent interactions in solid state, Inorganics 10 (2022) 151; DOI: 10.3390/inorganics10100151.
- I. S. Aliyarova, E. Yu. Tupikina, N. S. Soldatova, D. M. Ivanov, P. S. Postnikov, M. Yusubov, V. Yu. Kukushkin, Halogen bonding involving gold nucleophiles in different oxidation states, Inorg. Chem., 61 (2022); 10.1021/acs.inorgchem.2c01858.
- A.S. Novikov, D.S. Bolotin, Halonium, Chalconium, and Pnictonium Salts as Noncovalent Organocatalysts: A Computational Study on Relative Catalytic Activity, Org. Biomol. Chem., 2022, DOI: 10.1039/D2OB01415G.
- S. V. Baykov, D. M. Ivanov, S. O. Kasatkina, B. Galmés, A. Frontera, G. Resnati, V. Yu. Kukushkin, Stacking interactions: a supramolecular approach to upgrade weak halogen bond donors, Chem. Eur. J., 28 (2022); doi: 10.1002/chem.202201869.
- E. I. Chikunova, D. V. Dar’in, V. Yu. Kukushkin, A. Yu. Dubovtsev, Gold-catalyzed oxygen transfer to alkynylsulfones: a benign diazo-free route to 4-sulfonyl-1,3-oxazoles, Adv. Synth. Catal., 2022; doi: 10.1002/adsc.202200751.
- L. E. Zelenkov, D. M. Ivanov, I. A. Tyumentsev, Yu. A. Izotova, V. Yu. Kukushkin, N. A. Bokach, Structure-directing interplay between tetrel and halogen bonding in co-crystal of lead(II) diethyldithiocarbamate with tetraiodoethylene, Int. J. Mol. Sci., 23 (2022) 11870; doi: 10.3390/ijms231911870.
- M. A. Bondarenko, M. I. Rakhmanova, A. S. Novikov, M. N. Sokolov, S. A. Adonin, Bi- or trinuclear 2-iodobenzoate complexes of Znii: crystal structures and luminescence, Mendeleev Commun. 32 (2022) 585–587; DOI: 10.1016/j.mencom.2022.09.005.
- A. A. Babushkina, V. N. Mikhaylov, A. S. Novikov, V. N. Sorokoumov, M. A. Gureev, M. A. Kryukova, A. O. Shpakov, I. A. Balova, Synthesis, X-ray and DFT studies of 6-halo-3-(hydroxymethyl)cinnolin-4(1H)-ones, Chem. Heterocycl. Comp. 58 (2022) 432–437; DOI: 10.1007/s10593-022-03109-3.
- E. S. Starnovskaya, D. S. Kopchuk, A. F. Khasanov, O. S. Taniya, I. L. Nikonov, M. I. Valieva, D. E. Pavlyuk, A. S. Novikov, G. V. Zyryanov, O. N. Chupakhin, Synthesis and photophysical properties of α-(N-biphenyl)-substituted 2,2′-bipyridine-based push–pull fluorophores, Molecules 27 (2022) 6879; DOI: 10.3390/molecules27206879.
- I. N. Klyukin, A. V. Kolbunova, A. S. Novikov, A. P. Zhdanov, K. Y. Zhizhin, N. T. Kuznetsov, Theoretical Insight into B–C Chemical Bonding in Closo-Borate [BnHn−1CH3]2− (n = 6, 10, 12) and Monocarborane [CBnHnCH3]− (n = 5, 9, 11) Anions, Inorganics 10 (2022) 186; DOI: 10.3390/inorganics10110186.
- A. V. Nelyubin, I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, N. A. Selivanov, A. Y. Bykov, A. S. Kubasov, K. Y. Zhizhin, N. T. Kuznetsov, New Aspects of the Synthesis of closo-Dodecaborate Nitrilium Derivatives [B12H11NCR]− (R = n-C3H7, i-C3H7, 4-C6H4CH3, 1-C10H7): Experimental and Theoretical Studies, Inorganics 10 (2022) 196; DOI: 10.3390/inorganics10110196.
- P. Debnath, P. Debnath, M. Roy, L. Sieroń, W. Maniukiewicz, T. Aktar, D. Maiti, A. S. Novikov, T. K. Misra, Novel Organotin(IV) Complexes of 2-[4-Hydroxy-3-((2-hydroxyethylimino)methyl)phenylazo]benzoic Acid: Synthesis, Structure, Noncovalent Interactions and In Vitro Antibacterial Activity, Crystals 12 (2022) 1582; DOI: 10.3390/cryst12111582.
- V. V. Fedotov, M. I. Valieva, O. S. Taniya, S. V. Aminov, M. A. Kharitonov, A. S. Novikov, D. S. Kopchuk, P. A. Slepukhin, G. V. Zyryanov, E. N. Ulomsky, V. L. Rusinov, V. N. Charushin, 4-(Aryl)-Benzo[4,5]imidazo[1,2-a]pyrimidine-3-Carbonitrile-Based Fluorophores: Povarov Reaction-Based Synthesis, Photophysical Studies, and DFT Calculations, Molecules 27 (2022) 8029; DOI: 10.3390/molecules27228029.
- E. M. Lorits, E. A. Gubar, A. S. Novikov, Design of the Algorithm for Packaging of Water Molecules in a Fixed Volume, Symmetry 14 (2022) 2453; DOI: 10.3390/sym14112453.
- K. K. Geyl, S. V. Baykov, S. O. Kasatkina, P. Yu. Savko, V. P. Boyarskiy, Reaction of coordinated isocyanides with substituted N-(2-pyridyl)ureas as a route to new cyclometallated Pd(II) complexes, J. Organomet. Chem., 980–981 (2022), 122518. DOI: 10.1016/j.jorganchem.2022.122518.
- S. I. Presnukhina, M. V. Tarasenko, K. K. Geyl, S. O. Baykova, S. V. Baykov, A. A. Shetnev, V. P. Boyarskiy, Unusual formation of 1,2,4-oxadiazine core in reaction of amidoximes with maleic or fumaric esters, Molecules, 27 (2022), 7508. DOI: 10.3390/molecules27217508.
- A. A. Shetnev, A. S. Volobueva, V. A. Panova, V. V. Zarubaev, S. V. Baykov, Design of 4-substituted sulfonamidobenzoic acid derivatives targeting Coxsackievirus B3, Life, 12 (2022), 1832. DOI: 10.3390/life12111832.
- D. A. Polonnikov, M. V. Il'in, Ya. V. Safinskaya, I. S. Aliyarova, A. S. Novikov, D. S. Bolotin, (Pre)association as a Crucial Step for Computational Prediction and Analysis of the Catalytic Activity of Sigma-Hole Donating Organocatalysts, Org. Chem. Front., 2022, in press, DOI: 10.1039/D2QO01648F.
- N. S. Soldatova, V. V. Suslonov, D. M. Ivanov, M. S. Yusubov, G. Resnati, P. S. Postnikov, V. Yu. Kukushkin, Controlled halogen bond-involving assembly of double-sigma-hole donating diaryliodonium cations and ditopic arene sulfonates, Crystal Growth & Design, 22 (2022); doi: 10.1021/acs.cgd.2c01090.
- A. S. Novikov, Plethora of non-covalent interactions in coordination and organometallic chemistry are modern smart tool for materials science, catalysis, and drugs design, Int. J. Mol. Sci. 23 (2022) 14767; DOI: 10.3390/ijms232314767.
- L. K. Sadieva, I. S. Kovalev, O. S. Taniya, V. A. Platonov, A. S. Novikov, V. S. Berseneva, S. Santra, G. V. Zyryanov, B. C. Ranu, V. N. Charushin, Bola-type PEG-linked polyaromatic hydrocarbon-based chemosensors for the “turn-off” excimer fluorescence detection of nitro-analytes/explosives in aqueous solutions, Dyes and Pigments 210 (2023) 111014; 10.1016/j.dyepig.2022.111014.
- A. D. Sharapov, R. F. Fatykhov, I. A. Khalymbadzha, M. I. Valieva, I. L. Nikonov, O. S. Taniya, D. S. Kopchuk, G. V. Zyryanov, A. P. Potapova, A. S. Novikov, V. V. Sharutin, O. N. Chupakhin, Fluorescent pyranoindole congeners: synthesis and photophysical properties of pyrano[3,2-f], [2,3-g], [2,3-f], and [2,3-e]Indoles, Molecules 27 (2022) 8867; DOI: 10.3390/molecules27248867.
- A. S. Miroshnichenko, K. V. Deriabin, A. A. Rashevskii, V. V. Suslonov, A. S. Novikov, I. S. Mukhin, R. M. Islamova, Structural features of Eu3+ and Tb3+-bipyridinedicarboxamide complexes, Polymers 14 (2022) 5540; DOI: 10.3390/polym14245540.
- R. A. Popov, A. S. Mikherdov, V. P. Boyarskiy, Pyridine-2(1H)-thione as a bifunctional nucleophile in reaction with PdII and PtII aryl isocyanide complexes. Eur. J. Inorg. Chem., 2022 (2022), e202200217; DOI: 10.1002/ejic.202200217.
- Yu. A. Orekhova, A. S. Mikherdov, V. V. Suslonov, V. P. Boyarskiy. Coupling of thiazole-2-amines with isocyanide ligands in bis-(isocyanide) platinum complex: a new type of reactivity, Inorganics, 10 (2022), 221; DOI: 10.3390/inorganics10120221.
- V. Y. Mikshiev, P. M. Tolstoy, E. Yu. Tupikina, A. M. Puzyk, M. A. Vovk, Acid catalysis through N-protonation in undistorted carboxamides: improvement of amide proton sponge acylating ability, New J. Chem. 46 (2022) 16471–16484. DOI: 10.1039/d2nj02975h.
- B. G. Sokolov, V. V. Norin, E. A. Sidel’nikova, A. V. Kameshkov, E. V. Sladkovskaya, V. P. Boyarskiy, Cobalt catalysts for hydroformylation and carboalkoxylation: history and commercial prospects. Russ. J. Appl. Chem., 95 (2022), 631–645; DOI: 10.1134/S1070427222050020.
- K. K. Geyl, S. O. Baykova, P. A. Andoskin, V. V. Sharoyko, A. A. Eliseeva, S. V. Baykov, K. N. Semenov, V. P. Boyarskiy, Palladium(II) and platinum(II) deprotonated diaminocarbene complexes based on N-(2-pyridyl)ureas with oxadiazole periphery, Inorganics, 10 (2022), 247; DOI: 10.3390/inorganics10120247.
- A. S. Mazur, P. M. Tolstoy, K. Sotiriadis, 29Si NMR investigation of the effect of acetic and oxalic acids on Portland-limestone cement hydration, Mater. Sci. Forum, 1071 (2022) 247–252; DOI: 10.4028/p-73l5m1.
- Strashkov, D.M., Zavyalov, K.V., Sakharov, P.A., ...Khlebnikov, A.F., Novikov, M.S. Rhodium-catalyzed migrative annulation and olefination of 2-aroylpyrroles with diazoesters. Org. Chem. Front. 2022, 10, 506–513. DOI: 10.1039/D2QO01759H.
- Karcev, D.D., Efremova, M.M., Molchanov, A.P., ...Bunev, A.S., Khochenkov, D.A. Selective and Reversible 1,3-Dipolar Cycloaddition of 2-(2-Oxoindoline-3-ylidene)acetates with Nitrones in the Synthesis of Functionalized Spiroisoxazolidines. Int. J. Mol. Sci. 2022, 23, 12639. DOI: 10.3390/ijms232012639.
New resource saving processes based on organic reactions catalyzed by transition metal complexes
The objective of this project is the improvement of catalytic methods of organic chemistry for more rational resources using. Precatalysts structures and structure–activity relationships will be studied to achieve this goal, and then the detected patterns will be used to develop new resource saving catalyst systems. Homogeneous catalysis is in line with the so-called "green chemistry". Its purpose is more rational using of resources and reducing the harm caused by chemical processes to the environment. Among the principles of green chemistry one directly calls for chemists to give preference to catalytic processes, and a few (such as the minimization of energy consumption or use of more accessible starting compounds and the reduction of the synthetic steps number) indirectly linked to the development of catalytic techniques in organic synthesis.
The research will be concentrated in a few areas of homogeneous metal complexes catalysis, which are actively developing at the moment, namely:
- Synthesis of acyclic diamino carbene complexes of palladium(II) (Pd-ADC) and the study of their catalytic activity in many organic reactions;
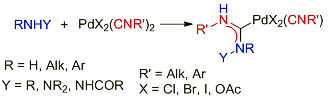
- Preparation of cobalt and nickel complexes and nanoparticles (including in situ), can catalyze the reduction and the substitution in the aryl chlorides, as well as studying their catalytic capabilities for utilization of persistent organic pollutants - polychlorinated biphenyls (PCB) - and the preparation on their basis precursors for electro polymers;
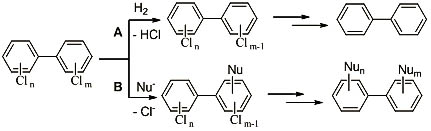
- Investigation of copper catalyzed C-C and C-N cross-couplings, including the reactions in an aqueous medium.