Department of Physical Chemistry
Plasmon-enhanced spectroscopy and bioimaging
Short URL for media go.spbu.ru/rgsolovyeva
Group Members
 |
Group Leader Elena V. SolovyevaPhD, associate professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Galina A. Chripunlead engineer This email address is being protected from spambots. You need JavaScript enabled to view it. | This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2088, 2093 |
 |
Aleksei N. SmirnovPhD student This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2090, 2093, 2008 |
 |
Vasilisa O. Svinkomaster student laboratory researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Alisa I. Shevchukmaster student research engineer This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Andrey I. Demenshinbachelor student laboratory researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Aleksei A. Smirnovbachelor student laboratory researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Simar F. Araslanovbachelor student This email address is being protected from spambots. You need JavaScript enabled to view it. |
Current Research Topics
| Development of optical labels and theranostics agents for therapeutic photodynamic and hyperthermal therapy based on gold nanoparticles | 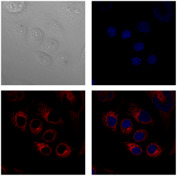 |
| Charge transfer processes between photoactive ligand and plasmonic nanoparticle, study of their mechanisms and role in surface-enhanced Raman scattering | 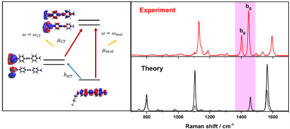 |
| Analytical applications of surface-enhanced Raman spectroscopy | 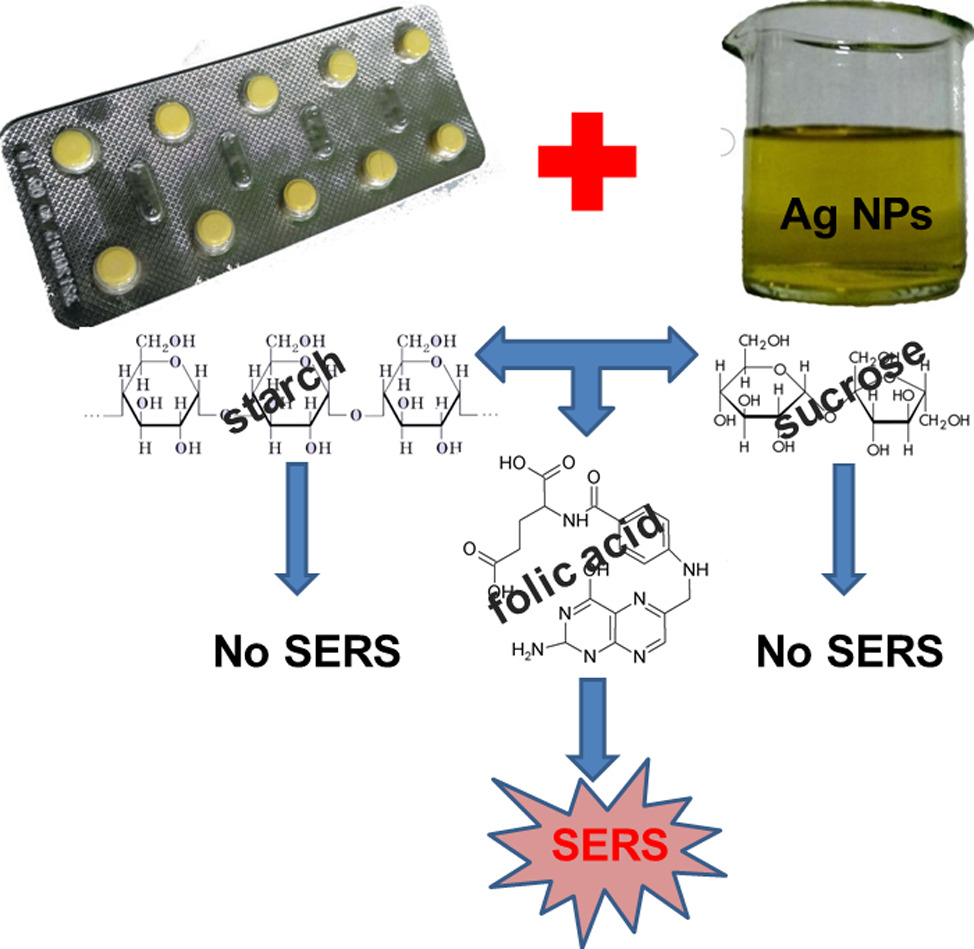 |
| Development of materials based on hydroxyapatite doped with metal nanoparticles for biomedicine and environmental engineering | 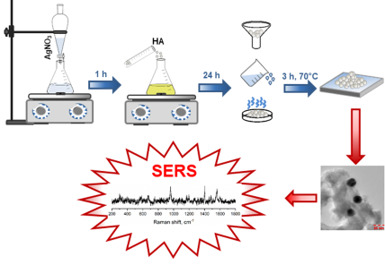 |
| Organic-Inorganic nanocomposites with controlled size and structure for optics and photonics |  |
Collaborations
- Imperial College London, Department of Chemical Engineering, prof. S. Kazarian group
- Sharif University of Technology, Department of Chemistry, prof. Z. Jamshidi group
- Almazov National Medical Research Centre
- Department of Laser Chemistry and Laser Material Science
- Department of Organic Chemistry
Grants, Scholarships and Awards
Grants and Projects
- SPBU Grant «Laboratory of plasmon-enhanced spectroscopy and bioimaging» (within the program «Foundation of Laboratory under leading young scientist leadership») 2022–2024
- RSF Grant 22-73-10052 «Multimodal plasmon tags for bioimaging and therapeutic hyperthermia» 2022-2025
- RFBR Grant 20-33-70034 «Self-organized plasmon structures based on metal nanoparticles and molecular bridges, promising as materials for optics and photonics» 2020–2021
- RSF Grant 17-73-10209 «Development of new organometallic substrates with "hot spots" promising for the use in highly efficient components of nanophotonics»,2017–2019
- Grant SPBU-Sharif University of Technology joint program «Effect of charge transfer in surface-enhanced Raman scattering: experimental and theoretical study» 2020
- Grant from Saint-Petersburg Government «Development of method for express analysis of water-soluble drugs using SERS spectroscopy to quality control» 2020
Students Scholarships and Awards
- Grant U.M.N.I.K «Photonics 2022» (Vasilisa Svinko)
- Youth Prize of Saint-Petersburg for 2021 in the nomination “Science and technology “(Aleksei N. Smirnov)
- The winners in the first competition dedicated to Andrei Pavlenko (Aleksei N. Smirnov, Vasilisa Svinko, Andrey Demenshin, Aleksei A. Smirnov)
- President scholarship for graduate students and young scientists (Aleksei N. Smirnov) 2022–2024
- Grant U.M.N.I.K «Photonics 2020» (Aleksei N. Smirnov)
- Start up SPBU 2020, 2 place (Aleksei N. Smirnov, Olga Odintsova)
Publications
- A.I. Shevchuk, V.O. Svinko, A.N. Smirnov, E.V. Solovyeva, SERS study of cyanine dyes: optimization of metal core and molecular label choice for plasmonic nanotags, Dyes and Pigments 216 (2023) 111329.
- V.O. Svinko, A.N. Smirnov, A.I. Shevchuk, A.I. Demenshin, A.A. Smirnov, E.V. Solovyeva, Comparative study of fluorescence core-shell nanotags with different morphology of gold core, Colloids and Surfaces B: Biointerfaces 2023, 226, 113306.
- A.N. Smirnov, S.F. Aslanov, D.V. Danilov, O.Y. Kurapova, E.V. Solovyeva, One-Pot Synthesis of Silica-Coated Gold Nanostructures Loaded with Cyanine 5.5 for Cell Imaging by SERS Spectroscopy, Nanomaterials 13 (2023) 1267.
- E.V. Solovyeva, O.V. Odintsova, V.O. Svinko, D.V. Makeeva, D.V. Danilov, Hydroxyapatite-nanosilver composites with plasmonic properties for application in surface-enhanced Raman spectroscopy, Materials Today Communications 35 (2023) 105908.
- E.V. Solovyeva, Z. Jamshidi, Observation of High-Order Overtones and Combinations in SERRS: The Essential Role on Elucidation of Chemical Mechanism, Journal of Physical Chemistry C 126 (2022) 12038–12043.
- Patent on invention 2774817 «Method for qualitative and quantitative determination of a biologically active substance in water-soluble medical products» issued on 23.06.2022.
- V.O. Svinko, A.I. Shevchuk, A N. Smirnov, D.V. Makeeva, E.V. Solovyeva, Gold nanostars-based labels for surface-enhanced Raman scattering imaging with red medical lasers, Optics and Spectroscopy 130 (2022) 1590–1595.
- E.V. Solovyeva, A.N. Smirnov, V.O. Svinko, A.S. Strelnikov, A.I. Shevchuk, S.G. Kazarian, Unraveling a role of molecular linker in nanoparticles self-organization by SERS spectroscopy: comparative study of three aromatic diamines, Colloids and Surfaces A 645 (2022) 128881.
Conferences
Our group actively participates in scientific conferences, for 2022 and the first half of 2023 we made more than 20 presentations, selected ones are indicated below.
- Russian conference and young scientists school on current problems of Raman spectroscopy "Raman Scattering-95", June 5-9 , 2023 Novosibirsk, Russia , oral and two poster talks (E.V. Solovyeva , A.I. Demenshin, V.O. Svinko);
- VIII International scientific conference of young scientists «Horizons of biopharmaceuticals», May 26, 2023 Kursk, Russia, oral talk (A.N. Smirnov);
- Scientific and practical conference with international participation «Granovsky readings — 2023», April 21-22, 2023 Saint-Petersburg, Russia, oral talk (E.V. Solovyeva)
- XXIX Russian Conference of Young Scientists «Current problems of biomedicine — 2023», March 29-30, 2023 Saint-Petersburg, Russia, oral talk (A.A. Smirnov);
- Young Scientists Congress, December 1-3, 2022 Sochi, oral talk (E.V. Solovyeva);
- The Seventh International Conference on Multifunctional, Hybrid and Nanomaterials , October 19-22, 2022 Jenoa, Italy, two poster talks (E.V. Solovyeva , .O. Svinko);
- VI International conference «Modern synthetic methodologies for the development of drugs and functional materials» (MOSM2022), November 7-11, 2022 Ekaterinburg, Russia, oral talk (A.I. Shevchuk);
- Saratov Fall Meeting 2022, September 26-30, 2022 Saratov, Russia, oral talk (A.N. Smirnov);
- 20th International Conference Laser Optics, June 20-24, 2022 Saint-Petersburg, Russia, oral talk (V.O. Svinko);
- 10th International Conference on Photonics, Optics and Laser Technology, February 10-11 , 2022 Porto, Portugal, oral talk (A.N. Smirnov);






Equipment and Methods
Our group has all the necessary resources for multiple step synthesis of nanoparticles and nanomaterials of inorganic and hybrid nature. Laboratory facilities include the equipment :
- Ultrasonic disperser 950-E (up to 950 W, treated volume of 0.5–600 mL, 1/12, 1/4, 5/12 inch probes)
- Centrifuge MPW-352 (speed up to 18000 rpm, rotors 12×2, 12×7, 6×50 mL)
- Rotary evaporator IKA RV 3 FLEX
- Vacuum drying cabinet SNVS-40/3.5 (temperature up to 350 °С, vacuum up to 1×10−4 mmHg)
- pH-meter Mettler Toledo S-220-Basic with micro and semi-micro pH electrodes
- Ultrasonic bath Sapphire 2.8 TTC
- Analytical balances, magnetic stirrers, pipettes





Our group has a clean area with a complete set of equipment (incubator, laminar flow cabinet, cell counter, centrifuge, plate spectrophotometer, etc.) necessary for cell culture and primary cell tests.
Applied protocols
- Cell viability assay with molecular compounds and nanoobjects
- Detection of reactive oxygen species in solutions and cell samples
- Detection of oxidative stress markers (malondialdehyde, protein carbonyl assay)
- Antioxidant enzymes activity assays (superoxide dismutase, peroxidase, catalase)
- Total antioxidant capacity assays with bioactive compounds and plant extracts (ABTS, DPPH, FRAP)
- Preparation of cell samples for Raman spectroscopy study
- Preparation of cell samples for fluorescence microscopy





Information for students
Bachelor and master students are welcome to work on their term papers and graduation theses. The particular topic will depend on the ongoing research projects of the lab.
Contacts: associate professor Elena Solovyeva, rooms 2088, 2093, This email address is being protected from spambots. You need JavaScript enabled to view it.
Department of Physical Chemistry
Lab #2094
Optical Senosrs Group
Short URL for media go.spbu.ru/rgpeshkova
Group Members
 |
Group Leader Maria PeshkovaPhD, associate professor This email address is being protected from spambots. You need JavaScript enabled to view it. | This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Andrey KalinichevPhD, assistant professor This email address is being protected from spambots. You need JavaScript enabled to view it. | This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Nadezhda Pokhvishchevapostgraduate student |
 |
Ivan Gryazevundergraduate student |
 |
Oleg Karpukhinundergraduate student |
 |
Vasiliy Syutkinundergraduate student |
 |
Tatiana Bulatovaundergraduate student |
Collaboration
- Group of chromatographic and electrophoretic methods of analysis (group leader Dr. Sc., full professor A. Kartsova)
- Group for laser deposition of metals from solutions (group leader PhD, associate professor I. Tumkin)
- IronBio and Vital Biosciences Inc. companies
Research Areas
Development of new operation concepts for polymeric optical sensors
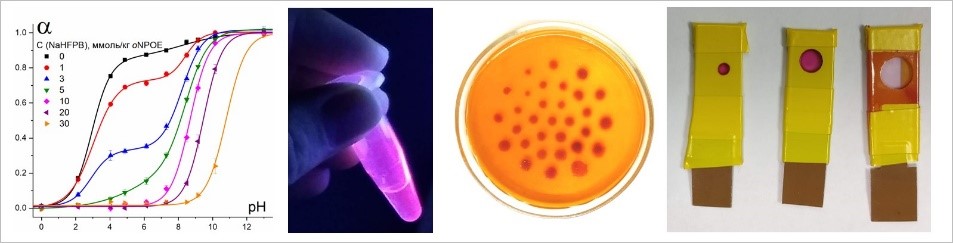
Theoretical formalization and prediction of optical response
Development of optical sensor-based devices
Advancing methods of digital color analysis and quantitative digital colorimetry
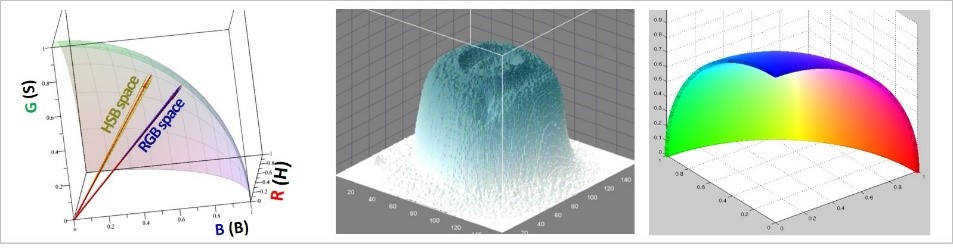
Electrochemical studies on sensing membranes
Modification of polymeric sensors with organic electrolytes

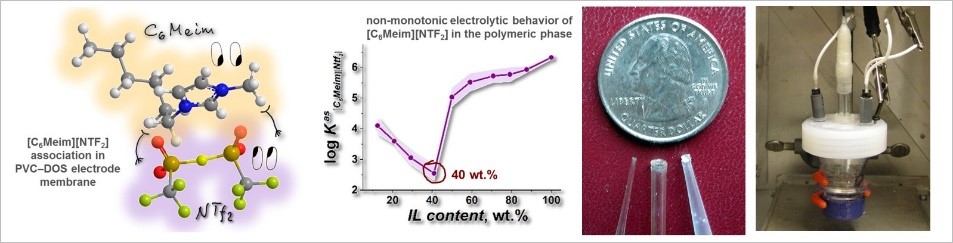
Ionic liquids in optical sensors

Development of calibration-free sensor arrays
Tailoring digital color analysis towards investigation of written artifacts
Current and recent projects
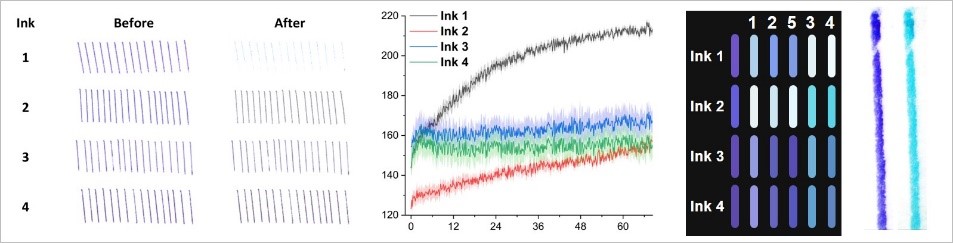
- RSF 22-23-00577, «Development of a method for the analysis and classification of ink compositions on paper substrates using selective extraction and digital color analysis» (A. Kalinichev, 2022–2023)
- RSF 20-73-10033, «Development and experimental validation of calibration-free optical sensors potentially applicable for early express-diagnostics of cystic fibrosis» (M. Peshkova, 2020–2023)
- RF President stipend, «Development of calibration-free analyzers based on optical sensors for multianalyte in situ analysis of human sweat electrolytes and pH» (A. Kalinichev, 2021–2023)
- Grant for young scientists at universities located in of St. Petersburg, Committee on Science and Higher Education of SPb Government (A.V. Kalinichev, 2021)
- RFBR 19-33-90279, «Generalization of the theory of polymeric optochemical sensor response involving electrical boundary potential as a universal instrument for controlling sensor characteristics» (A. Kalinichev, 2019–2021)
- RSF 18-73-00109 «Miniaturized multianalyte optochemical platform for autonomous in situ monitoring of hydroponic nutrient solutions» (M. Peshkova, 2018–2020)
Participation in scientific and educational events
- сonducting a project-oriented educational session for schoolchildren in chemistry at the Sirius educational center (Sochi) (2018–2021)
- participation in the methodical committee of the St. Petersburg Olympiad in Chemistry, 2018–2022 (A. V. Kalinichev)
- 4th International Caparica Conference on Chromogenic and Emissive Materials (Portugal, 2020)
- International Meeting on Chemical Sensors “Matrafured” (Visegrad, Hungary, 2017, 2019, 2022)
- All-Russian Conference on Electrochemical Methods of Analysis “EMA” (2008, 2012, 2016)
- Meetings of the International Society of Electrochemistry (2012, 2014)
- 4th International Conference on Biosensor Technologies (Lisbon, Portugal, 2015)
- International Conference for Young Scientists in Chemistry “Mendeleev” (SPb, Russia, 2013, 2014, 2015, 2019, 2021)
- 14th International Conference on Electronic and Optical Sensors (Gdansk, Poland, 2016)
- International Scientific Conference for Undergraduate and Graduate Students and Young Scientists "Lomonosov" (Moscow, Russia, 2016, 2017, 2019)
- XXXII European Conference on chemical sensors “Eurosensors” (Graz, Austria, 2018)
- XXI Mendeleev Congress on General and Applied Chemistry (SPb, Russia, 2019)
Awards
- Prize of the Government of St. Petersburg in the field of scientific and pedagogical activity (M.A. Peshkova, 2017, 2020, 2022)
- Grant for students of universities located on the territory of St. Petersburg, Committee on Science and Higher Education of SPb Government (A.V. Kalinichev, 2018, 2020; N.V. Pokhvishcheva, 2020, 2022)
- Analit-Shimadzu Scholarship for Master's Students (N.V. Pokhvishcheva, 2020)
- V. Potanin Scholarship (N.Yu. Tyuftyakov, 2020)
- Grant from the Academy of Finland (A.V. Kalinichev, 2019)
- 1st degree Diploma at "Mendeleev 2019" (A.V. Kalinichev, 2019)
- 1st and 2nd degree Diplomas at the International Scientific Conference for Undergraduate and Graduate Students and Young Scientists "Lomonosov" (A.V. Kalinichev, 2017; N.V. Pokhvishcheva, 2017, 2019; I.S. Prozherin, 2022)
- Diploma at III competition of business-cases «Changellenge >> Danone One-Day Lab» (A.V. Kalinichev, 2017)
- Diplomas of the SPbU start-up competition (1st degree, A.V. Kalinichev, 2015; 2nd degree, D.I. Dekina, 2017; finalist diploma, A.V. Kalinichev, 2018)
- Grant of the start-up competition “UMNIK” (A.V. Kalinichev, 2015–2016)
- 1st degree Diploma in the tournament of innovative projects in the field of chemistry and materials science "Mendeleev 2015" (A.V. Kalinichev, 2015)
Publications
Selected Publications
- A.V. Kalinichev, A.V. Kravchenko, I.P. Gryazev, A.A. Kechin, O.R. Karpukhin, E.M. Khairullina, L.A. Kartsova, A.G. Golovkina, V.A. Kozynchenko, M.A. Peshkova, I.I. Tumkin, Classification of ballpoint pen inks based on selective extraction and subsequent digital color and cluster analyses, Analyst 147 (2022) 3055–3064; IF 5.227 (2021) https://doi.org/10.1039/D2AN00482H
- N.V. Pokhvishcheva, E.K. Gigiadze, A.V. Kalinichev, A.V. Ievlev, K.V. Tyutyukin, M.A. Peshkova, Chronopotentiometric Evaluation of Ionization Degree and Dissociation Constant of Imidazolium‐Based Ionic Liquid [C6Meim][NTf2] in Polymeric Plasticized Membranes, Membranes (MDPI) 12(2) (2022) 130; IF 4.562 (2021) https://doi.org/10.3390/membranes12020130
- N.Y. Tiuftiakov, A. V. Kalinichev, N. V. Pokhvishcheva, M.A. Peshkova, Digital color analysis for colorimetric signal processing: Towards an analytically justified choice of acquisition technique and color space, Sensors and Actuators B 344 (2021) 130274; IF 7.460 (2020) https://doi.org/10.1016/j.snb.2021.130274
- N.Y. Tiuftiakov, A. V. Kalinichev, E. Khairullina, E.K. Gigiadze, M.A. Peshkova, I.I. Tumkin, Simple and Cost-Efficient Classification of Ballpoint Pen Inks Using Digital Color Analysis, Anal. Chem. 93 (2021) 5015–5019; IF 6.986 (2020) https://doi.org/10.1021/acs.analchem.0c05334
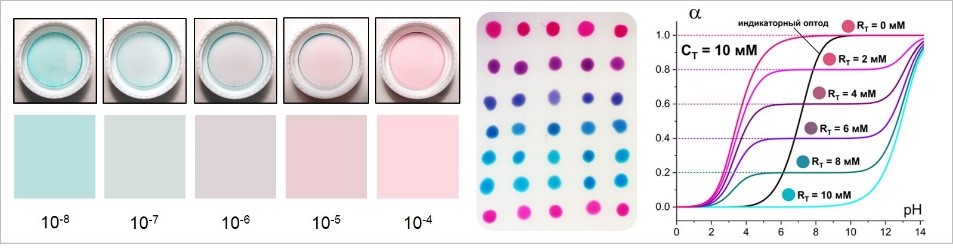
- A. V. Kalinichev, N. V. Pokhvishcheva, M.A. Peshkova, Influence of Electrolyte Coextraction on the Response of Indicator-Based Cation-Selective Optodes, ACS Sensors. 5 (2020) 3558–3567; IF 7.711 (2020) https://doi.org/10.1021/acssensors.0c01747
- N.Y. Tiuftiakov, A. V. Kalinichev, I. V. Rudenko, E.A. Bessonova, L.A. Kartsova, M.A. Peshkova, pH-dependent distribution of the indicator dye tetrabromophenolphthalein ethyl ester between aqueous solution and plasticized polymeric phase: Predicting the lifetime of ion-selective optical sensors, Colloid Interface Sci. Commun. 37 (2020) 100295; IF 4.914 (2020) https://doi.org/10.1016/j.colcom.2020.100295
- Pokhvishcheva, N. V., Peshkova, M. A. Ionic Liquids as Plasticizers for Optodes, Moscow Univ. Chem. Bull. (2020) 75(2) 115-120 https://doi.org/10.3103/S002713142002011X
- Polikarpova D., Makeeva D., Kolotilina N., Dolgonosov A., Peshkova M., Kartsova L. Nanosized cation exchanger for the electrophoretic separation and preconcentration of catecholamines and amino acids, Electrophoresis (2020) 41 (12) 1031-1038; IF 3.535 (2020) https://doi.org/10.1002/elps.201900416
- Kartsova, L.; Moskvichev, D.; Bessonova, E.; Peshkova, M. Imidazolium Ionic Liquids in Microemulsion Electrokinetic Chromatography for Separation of Polyphenol Antioxidants, Chromatographia (2020) 83(8) 1001-1008; IF 2.044 (2020) https://doi.org/10.1007/s10337-020-03921-z
- A. V. Kalinichev, N. V. Pokhvishcheva, M.A. Peshkova, Novel color standards for digital color analysis of optochemical sensor arrays, Talanta. 197 (2019) 638–644; IF 6.057 (2020) https://doi.org/10.1016/j.talanta.2019.01.063
- A. V. Kalinichev, N. V. Pokhvishcheva, M.A. Peshkova, Significant Reduction of Analysis Time with Bulk Sensors Operating in Nonequilibrium Mode, Anal. Chem. 91 (2019) 5362–5370; IF 6.986 (2020) https://doi.org/10.1021/acs.analchem.9b00459
- Galiullin, T. M., Pokhvishcheva, N. V., Kalinichev, A. V., Peshkova, M. A. Evaluation of Ionic Liquids Based on Amino Acid Anions for Use in Liquid‐junction Free Reference Electrodes, Electroanalysis 31 (2019), 1708-1718; IF 3.223 (2020) https://doi.org/10.1002/elan.201900125
- Dekina, D.I., Kalinichev, A.V., Pokhvishcheva, N.V., Peshkova, M.A., Mikhelson, K.N. Effects of quantitative composition of the sensing phase in the response of ionophore-based optical sensors, Sensors and Actuators B 277 (2018) 535-543; IF 7.460 (2020) https://doi.org/10.1016/j.snb.2018.09.018
- Kalinichev A.V., Peshkova M.A., Pokhvishcheva N.V., Mikhelson K.N. Ion-selective optical sensors: a new look at well-established techniques of signal acquisition, Proceedings MDPI, 2 (2018) 825, https://doi.org/10.3390/proceedings2130825
- Andrey V. Kalinichev, Ana Frosinyuk, Maria A. Peshkova, Konstantin N. Mikhelson, The Impact of Ion Association in the Optode Phase to the Dynamic Range and the Sensitivity of the Response of Ion-Selective Bulk Optodes, Sensors and Actuators B 249 (2017) 123-130; IF 7.460 (2020) https://doi.org/10.1016/j.snb.2017.03.088
Teaching
Elective courses
- Chemical Sensors (in Russian, Chemistry, undergraduate, 5th semester)
- Chemical Sensors (in Russian, Chemistry, master program, 2nd semester)
- Chemical Sensors (in English, Chemistry, master program, 3rd semester)
- Biomedical Applications of Optical Sensors (in English, Chemistry, master program, 3rd semester)
Topics of coursework projects
physical chemistry, 2nd year
- Influence of organic electrolytes in the composition of optical sensors on the colorimetric response
- Investigation of an interplay between the external potential and the colorimetric response of ion-selective optical sensors
- In situ electrochemical study of the electrolytic properties of organic salts in the composition of polymeric sensor membranes
- Organic electrolytes in the composition of polymeric membranes of potentiometric sensors
- Evaluation of partition coefficients of electrolytes between the plasticized polymeric membrane and the aqueous phase
analytical chemistry, 2nd year
- Optimization of the optode-based sensor platform for in situ analysis of sweat
- Exploring the possibilities of chromoionophore-based optical sensors for creating calibration-free sensor arrays
- Classification and aging dynamics of ball-pen inks by means of digital color analysis
- Comparison of changes in chemical nature and color of coloring components in ballpoint pen inks under UV radiation
Department of Analytical Chemistry
Membrane Materials and Membrane Separation Methods Scientific Group
Short URL for media go.spbu.ru/rgpenkova
Team Members
Research Team
 |
Group Leader Penkova AnastasiaDoctor of Science, Professor This email address is being protected from spambots. You need JavaScript enabled to view it. WoS ResearcherID: J-3228-2013 | Scopus AuthorID: 14062446100 |
 |
Dmitrenko MariiaPhD, Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. WoS ResearcherID: I-8143-2014 |
 |
Kuzminova AnnaPhD, Assistant This email address is being protected from spambots. You need JavaScript enabled to view it. WoS ResearcherID - D-7870-2019 |
 |
Plisko TatianaPhD, Leading researcher This email address is being protected from spambots. You need JavaScript enabled to view it. WoS ResearcherID: R-7414-2018 |
 |
Burt EkaterinaPhD, Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. WoS ResearcherID: AAT-6749-2020 |
 |
Dubovenko RomanResearcher This email address is being protected from spambots. You need JavaScript enabled to view it. Wos ResearcherID: JFS-6534-2023 |
 |
Kariakina AnnaMaster student, 2 year, Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Sushkova KseniaMaster student, 2 year, Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Myznikov DanilaBachelor student, 3 year, Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. | This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Puzikova MargaritaBachelor student, 3 year, Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Mikulan AnnaBachelor student, 3 year, Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Mikhailovskaya OlgaBachelor student, 3 year, Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Korovina AlexandraBachelor student, 3 year This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Karimova OlesyaBachelor student, 2 year This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Salomatin KirillBachelor student, 2 year This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Kudrina SeverinaBachelor student, 2 year This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Rakovskaya NadezhdaBachelor student, 1 year This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Stepanova AnastasiaBachelor student, 3 year This email address is being protected from spambots. You need JavaScript enabled to view it. |
Alumni
 |
Zolotarev MariiaPhD This email address is being protected from spambots. You need JavaScript enabled to view it. WoS ResearcherID: N-1250-2016 |
 |
Loshinina JuliaGraduate student, 2023 This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Lyamin VladislavGraduate student, 2023 This email address is being protected from spambots. You need JavaScript enabled to view it. WoS ResearcherID: AAF-6532-2021 |
 |
Chepeleva AnastasiiaGraduate student, 2023 This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Pletneva MariiaPostgraduate student, 2022 This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Loginova EugeniiaUndergraduate student, 2022 |
 |
Surkova ViktoriaUndergraduate Student, 2021 This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Atta RamadanPostgraduate Student, 2021 This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Ochkalova SofiaUndergraduate Student, 2019 year |
 |
Vasin SemenUndergraduate Student 2018 year |
 |
Gubina EvgeniaBachelor student 4 year |
 |
Savon NadejdaGraduate Student 2016 year |
 |
Polyakov EvgeniyGraduate Student 2015 year |
 |
Krasnova ViktoriaUndergraduate Student 2012 year |
 |
Oshin EvgeniyGraduate Student 2010 year |
 |
Gavrilova ViktoriaGraduate Student 2009 year |
Collaboration
- Reactions and Processes Laboratory, Membrane Group of Professor Denis Roizard, University of Lorraine, Nancy, France;
- Scientific Group of Christian Serre, Institut Lavoisier de Versailles, Versailles, France;
- Scientific Group of Erkki Lähderanta, LUT University, Lappeenranta, Finland;
- Scientific Group of Vice-Chancellor Prof. Sabu Thomas, International & Inter-University Centre for Nanoscience and Nanotechnology, Mahatma Gandhi University Kerala, India;
- Membrane Processes Laboratory of Academician of the National Academy of Sciences of Belarus Prof. Bildyukevich Alexander, Institute of Physical and Organic Chemistry of the National Academy of Sciences of Belarus, Minsk, Belarus;
- Scientific Group of Ilya Vorotyntsev, Nizhny Novgorod State Technical University n.a. R.E. Alekseev, Nizhny Novgorod, Russia;
- Polymeric Membranes Laboratory of Prof. Alexei Volkov, A.V.Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences (TIPS RAS), Moscow, Russia;
- Scientific Group of Prof. Rongxin Su, Tianjin University, Tianjin, China;
- Scientific Group of Prof. Daniel Pasquini, Institute of Chemistry, Federal University of Uberlândia, Uberlandia, Brazil;
- Scientific Group of Prof. Maya Jacob John, Council for Scientific & Industrial Research (CSIR), Pretoria, South Africa;
- Scientific Group of Prof. M.J. Hato, University of Limpopo, Limpopo, South Africa.
Fields of Scientific Interests
- Membrane materials: development, preparation, structure, transport properties;
- Membrane processes: pervaporation, ultrafiltration, nanofiltration, gas separation;
- Thermodynamics and kinetics of nonequilibrium processes.
Briefly about research
Polymer membranes, the surface and volume of which are modified by various inorganic and organic particles are of significant interest, both fundamentally and for the development of the foundations of membrane technology, its application in various fields of industry (petrochemical, medical, pharmaceutical, food and others), creation of environmentally friendly, energy- and resource-saving technologies. The development of new using particles of various physical and chemical nature is a recognized and promising area of nanotechnology. The scientific group develops and studies new polymer membrane materials (non-porous and porous) with a functional purpose using various physical and chemical methods of analysis. These membranes are used in various membrane processes, such as pervaporation, nanofiltration, gas separation and ultrafiltration for the separation of industrially important liquid and gas mixtures. Currently, thermodynamic and kinetic studies of membrane processes, as well as the related search for new membrane materials, are ongoing at the Department of Analytical Chemistry.
In the scientific group, a series of studies was carried out devoted to the development of methods for obtaining membranes modified with carbon particles and their characterization. Part of the research was carried out and published jointly with the Nobel laureate G. Kroto.
Publications
Selected publications
Dmitrenko Maria, Kuzminova Anna, Zolotarev Andrey, Markelov Denis, Komolkin Andrey, Loginova Evgenia, Plisko Tatiana, Burts K., Bildyukevich Alexandr, Penkova Anastasia. Modification strategies of polyacrylonitrile ultrafiltration membrane using TiO2 for enhanced antifouling performance in water treatment, Separation and Purification Technology, 286, 120500 (2022). DOI 10.1016/j.seppur.2022.120500.
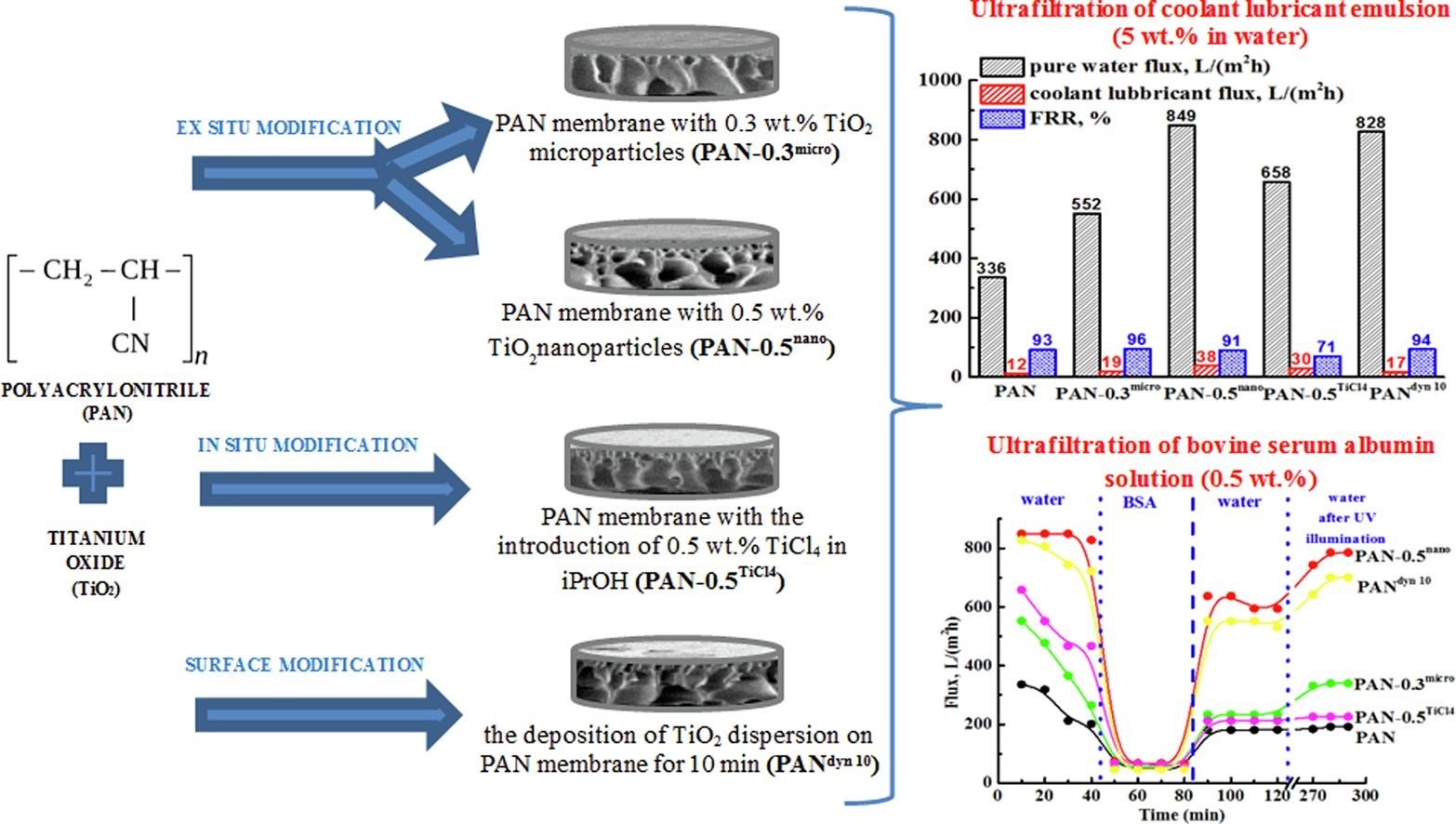
The active application of ultrafiltration in various industries requires the development of novel membranes with tailored properties and good fouling resistance. This work is devoted to the improvement of ultrafiltration properties of polyacrylonitrile (PAN) membranes by various TiO2 modification approaches: (1) ex situ method - the introduction of pre-formed micro- or nanoparticles; (2) in situ method - the formation of TiO2 particles in the casting solution; and (3) surface modification method - dynamic deposition of TiO2 on the membrane surface. The effect of the various TiO2 immobilization techniques on the structure of PAN membranes was studied by scanning electron and atomic force microscopies, and the contact angle measurements. The introduction of TiO2 particles improved membrane performance and antifouling stability under UV irradiation in ultrafiltration of industrially important feeds - bovine serum albumin solution (BSA) and coolant lubricant emulsion. The affinity to water of TiO2-modified PAN membrane was confirmed by atomistic molecular dynamics simulations, swelling experiments, and calorimetric study of wetting. PAN membrane with 0.5 wt% TiO2 nanoparticles had the optimal transport characteristics and improved surface self-cleaning ability after UV irradiation: pure water, coolant lubricant, and BSA fluxes (849, 38, and 68 L/(m2h), respectively), and flux recovery ratio after UV-illumination (95%).
Dmitrenko M.E., Kuzminova A.I., Zolotarev A.A., Korniak A.S., Ermakov S.S., Su R., Penkova A.V., Novel mixed matrix membranes based on polyelectrolyte complex modified with fullerene derivatives for enhanced pervaporation and nanofiltration. Separation and Purification Technology 2022, 298, 121649. 10.1016/j.seppur.2022.121649.
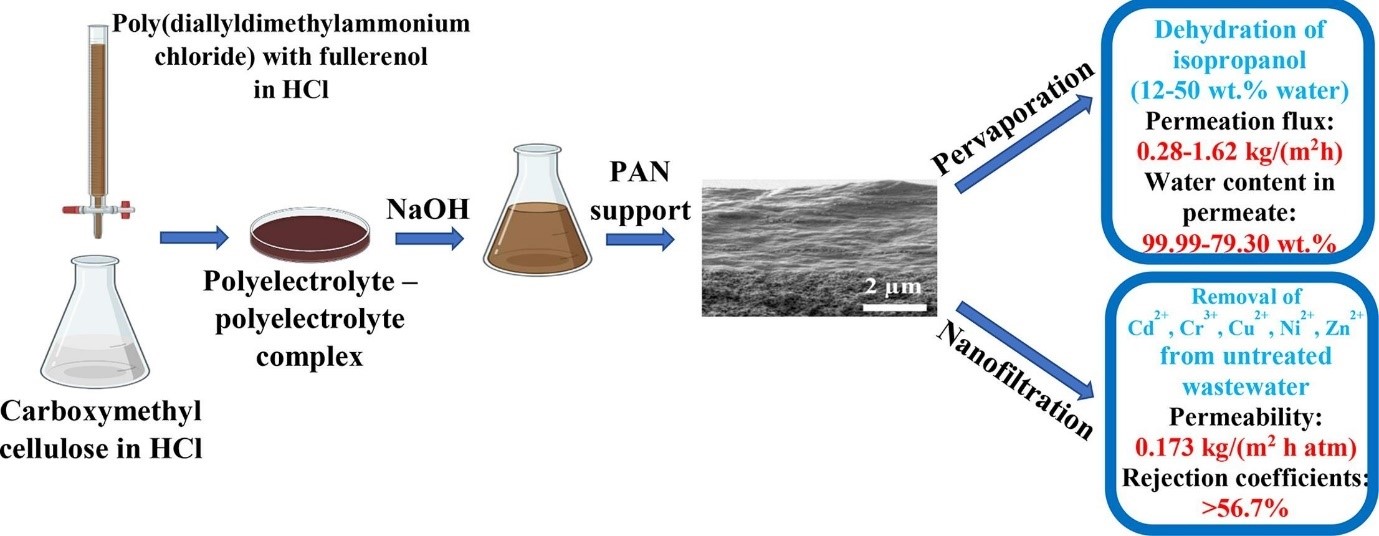
Solution-processable polyelectrolyte complex (PEC) modified with various water-soluble fullerene derivatives (fullerenol, carboxyfullerene, fullerene derivative with L-arginine) were synthesized by using sodium carboxymethyl cellulose (CMC) and poly(diallyldimethylammonium chloride) (PDADMAC) for the creation of novel supported mixed matrix membranes for enhanced pervaporation and nanofiltration. The optimal preparation conditions and membrane composition were found. The structural characteristics and physicochemical properties of PEC-based membranes were analysed by Fourier-transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), scanning electron (SEM) and atomic force (AFM) microscopies, thermogravimetric analysis (TGA), contact angle measurements and swelling experiments. The developed membranes were tested in pervaporation dehydration of isopropanol (12–50 wt% water) and, for the first time, in nanofiltration of heavy metals (model solutions and wastewater from galvanic production). Optimal transport characteristics were possessed by a supported membrane with a selective layer based on PEC-fullerenol (4%) composite: improved permeation flux of 0.28–1.62 kg/(m2h) and 99.99–79.30 wt% water in permeate in pervaporation dehydration of isopropanol (12–50 wt% water) at 22 °C, and 2.5 times improved permeability at a high rejection coefficients in nanofiltration of heavy metals compared to the pristine CMC membrane, which indicated its promise industrial application for water purification.
Kuzminova Anna I., Dmitrenko Mariia E., Poloneeva Daria Y., Selyutin Artem A., Mazur Anton S., Emeline Alexei V., Mikhailovskii Vladimir Y., Solovyev Nikolay D., Ermakov Sergey S.,.Penkova Anastasia V, Sustainable composite pervaporation membranes based on sodium alginate modified by metal organic frameworks for dehydration of isopropanol // Journal of Membrane Science, 626, 119194 (2021). doi: 10.1016/j.memsci.2021.119194
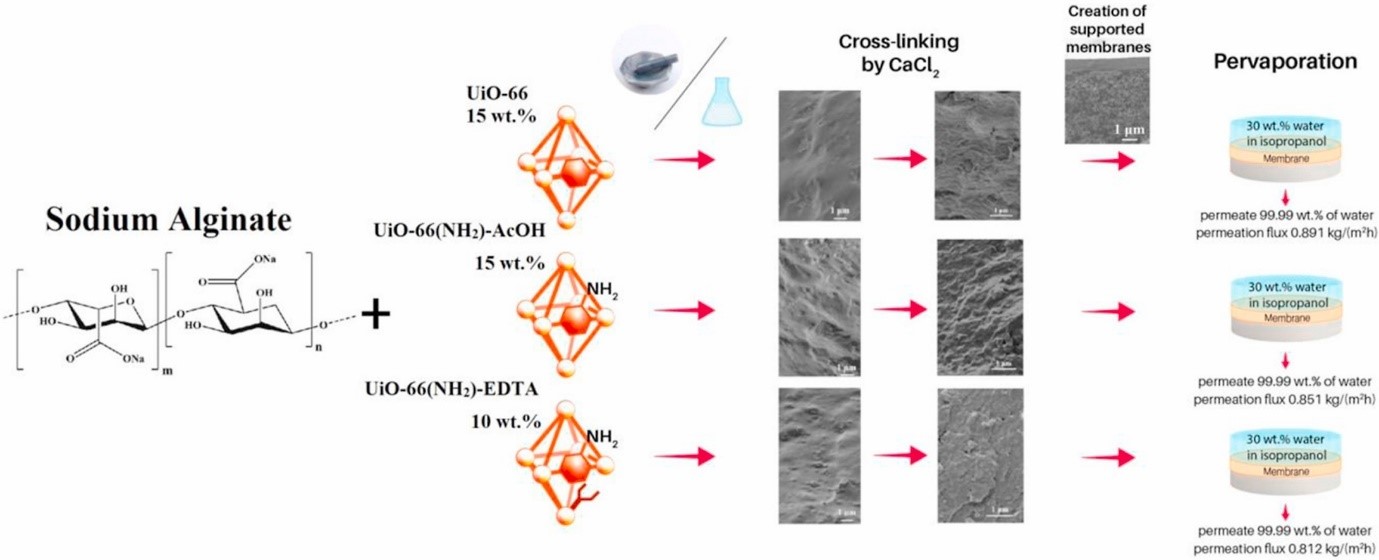
Novel dense and supported (polyacrylonitrile substrate) mixed matrix membranes based on biopolymer sodium alginate (SA), modified by Zr-MOFs were developed to improve pervaporation dehydration properties of a parent SA membrane. The following Zr-MOFs were synthesized and tested as modifiers: unmodified UiO-66 and modified UiO-66(NH2)-AcOH and UiO-66(NH2)-EDTA. Two kinds of mixed matrix membranes were developed: without additional treatment and cross-linked with calcium chloride. The synthesized Zr-MOFs nanoparticles and developed SA and SA-Zr-MOFs membranes were studied using Fourier-transform infrared spectroscopy, nuclear magnetic resonance, scanning electron microscopy, surface area measurement, atomic force microscopy, X-ray diffraction analysis, thermogravimetric analysis, and swelling experiments. Dense and supported membranes were tested for their transport properties in the pervaporation dehydration of isopropanol (12, 30 wt% water for the untreated membranes and 12–100 wt% water for the cross-linked membranes). The best transport properties (dehydration of water/isopropanol mixtures at 22 °C) were demonstrated by a supported cross-linked membrane, containing 15 wt% of UiO-66: permeation flux 0.47–3.38 kg/(m2h), water content in permeate 99.9-97.5 wt%.
Mariia Dmitrenko, Vladislav Liamin, Erkki Lahderanta, Sergey Ermakov, Anastasia Penkova, Mixed matrix membranes based on sodium alginate modified by fullerene derivatives with L-amino acids for pervaporation isopropanol dehydration. // Journal of Materials Science (2021), 56, 7765–7787. doi:10.1007/s10853-021-05791-8
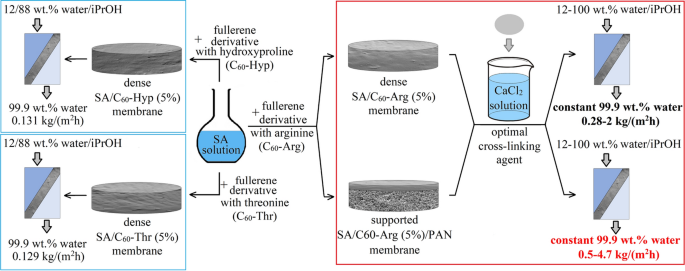
Biopolymer sodium alginate (SA) is actively used as a green membrane material. To improve the pervaporation properties of the SA membrane in the isopropanol dehydration, different water-soluble fullerene derivatives with L-amino acids (threonine, hydroxyproline, and arginine) were used. In this study, fullerene–arginine derivative (C60-Arg) was shown to be an optimal filler acting both as a modifier and a cross-linking agent for SA. Different cross-linking agents (phosphoric and citric acids, calcium chloride) were tested for dense membrane cross-linking to reinforce the membranes for the separation of diluted solutions. Two types of membranes based on SA and SA/C60-Arg (5%) were developed: dense and supported on polyacrylonitrile substrate. The structural features of obtained membranes were investigated by the following methods: FTIR spectroscopy, scanning electron (SEM), and atomic force (AFM) microscopies. The optimal transport properties in dehydration of isopropanol (12–100 wt.% water) were found for the cross-linked SA/C60-Arg (5%) membrane supported on PAN substrate. The following parameters were obtained: 0.5–4.7 kg/(m2h) permeation flux and constant 99.99 wt.% water content in the permeate.
Anastasia V.Penkova, Anna I. Kuzminova, Mariia E. Dmitrenko, Victoria A. Surkova, Vladislav P. Liamin, Denis A. Markelov, Andrei V. Komolkin, Daria Y. Poloneeva, Anastasia V. Laptenkova, Artem A. Selyutin, Anton S. Mazur, Alexei V. Emeline, Sabu Thomas, Sergey S. Ermakov. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66(NH2)-EDTA for highly efficient methanol isolation. // Separation and Purification Technology (2021), 118370. doi:10.1016/j.seppur.2021.118370
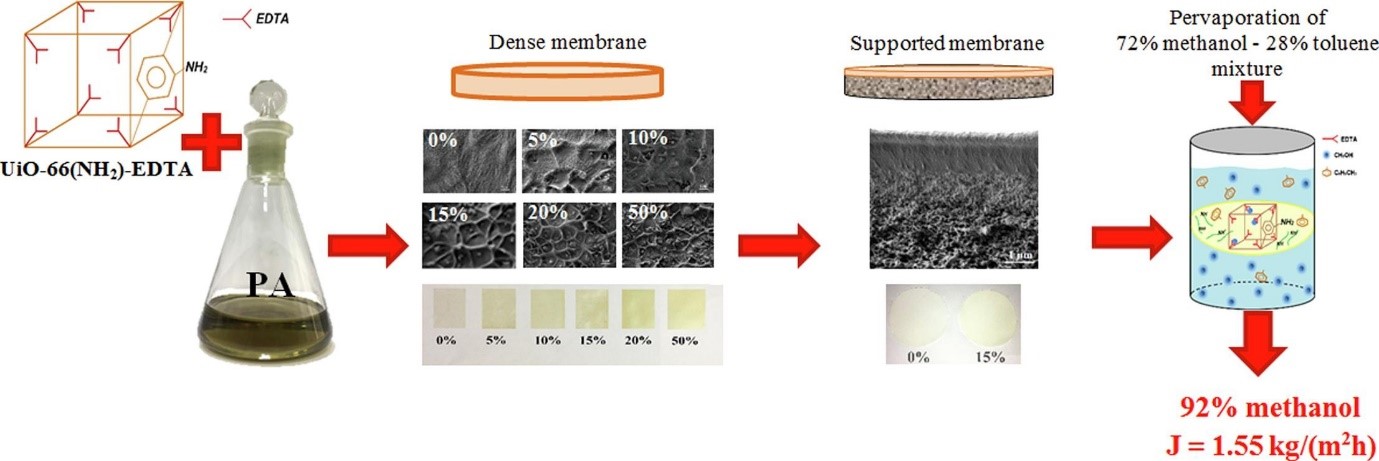
As a rule, the polymeric membranes have low permeability in separation of low molecular weight components. In spite of this fact, the membrane processes have significant advantages compare with conventional technologies, in particular, low energy consumption and environmental friendliness. To improve transport properties of the polymer membrane their modification should be carried out. In the present work, the development of highly methanol-permeable pervaporation membranes based on poly-m-phenylene isophthalamide (PA) is achieved by two strategies: (i) modification of PA by novel synthesized and characterized highly stable metal–organic framework UiO-66(NH2)-EDTA particles and (ii) development of supported membranes with thin selective layer on the regenerated cellulose substrate. First time the composite structure has been simulated: atomistic molecular dynamics simulations demonstrate the partial penetration of polymer inside the modifier and confirms the nature of the interaction between polymer and modifier assessed by spectroscopic methods. The optimal characteristics in respect of industrial use are obtained for supported PA/UiO-66(NH2)-EDTA (15%) membrane: 1.55 kg/(m2h) permeation flux and 93.1 wt% methanol in the permeate for the separation of azeotropic methanol/toluene mixture.
Dmitrenko M., Zolotarev A., Plisko T., Burts K., Liamin V., Bildyukevich A., Ermakov S., Penkova A., Effect of the Formation of Ultrathin Selective Layers on the Structure and Performance of Thin-Film Composite Chitosan/PAN Membranes for Pervaporation Dehydration // Membranes (2020), 10, 153. doi:10.3390/membranes10070153.
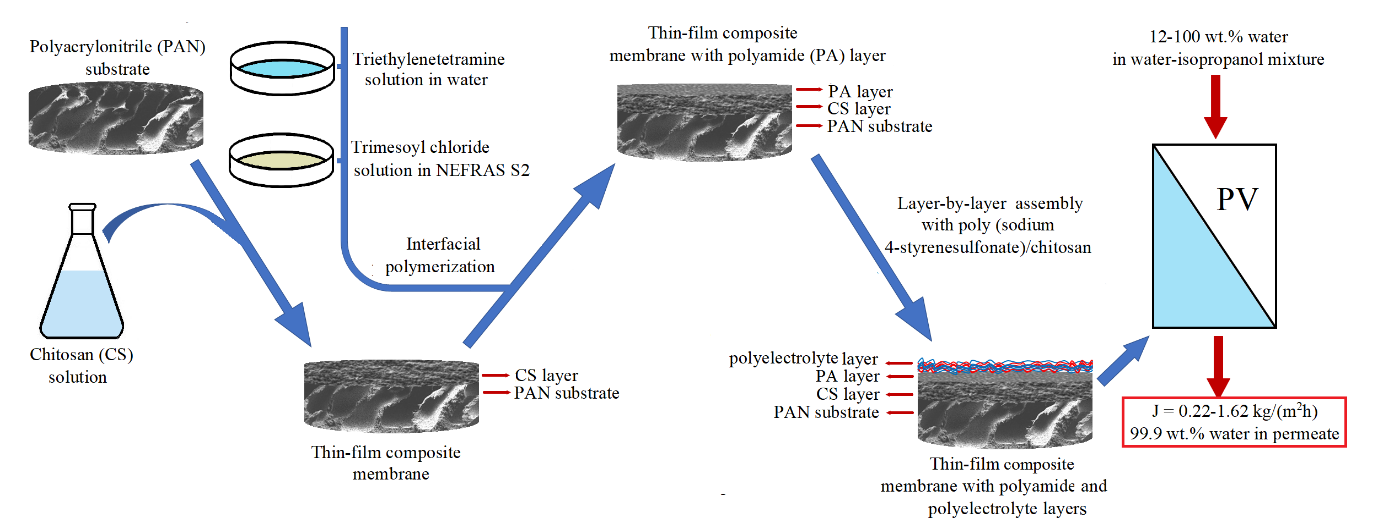
The aim of the study is to improve the performance of thin-film composite (TFC) membranes with a thin selective layer based on chitosan (CS) via different approaches by: (1) varying the concentration of the CS solution; (2) changing the porosity of substrates from polyacrylonitrile (PAN); (3) deposition of the additional ultrathin layers on the surface of the selective CS layer using interfacial polymerization and layer-by-layer assembly. The developed membranes were characterized by different methods of analyses (SEM and AFM, IR spectroscopy, measuring of water contact angles and porosity). The transport characteristics of the developed TFC membranes were studied in pervaporation separation of isopropanol/water mixtures. It was found that the application of the most porous PAN-4 substrate with combination of formation of an additional polyamide selective layer by interfacial polymerization on the surface of a dense selective CS layer with the subsequent layer-by-layer deposition of five bilayers of poly (sodium 4-styrenesulfonate)/CS polyelectrolyte pair led to the significant improvement of permeance and high selectivity for the entire concentration feed range. Thus, for TFC membrane on the PAN-4 substrate the optimal transport characteristics in pervaporation dehydration of isopropanol (12–90 wt.% water) were achieved: 0.22–1.30 kg/(m2h), 99.9 wt.% water in the permeate
2024
- Dmitrenko M., Kuzminova A., Dubovenko R., Mikulan A., Puzikova M., Selyutin A., Mazur A., Ermakov S., Su R., Penkova A., Carboxymethyl cellulose/Zn-based metal organic frameworks membranes for pervaporation-assisted esterification reactor // Separation and Purification Technology. 2024, 332, 125720. https://doi.org/10.1016/j.seppur.2023.125720
2023
- Dmitrenko M., Kuzminova A., Zolotarev A., Selyutin A., Ermakov S., Penkova A. Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions // Polymers 2023, 15, 1341. https://doi.org/10.3390/polym15061341
- Kuzminova A., Dmitrenko M., Zolotarev A., Markelov D., Komolkin A., Dubovenko R., Selyutin A., Wu J., Su R., Penkova A. Novel Mixed Matrix Membranes Based on Poly(vinylidene fluoride): Development, Characterization, Modeling // Polymers 2023, 15, 1222. https://doi.org/10.3390/polym15051222
- Plisko T., Burts K., Penkova A., Dmitrenko M., Kuzminova A., Ermakov S., Bildyukevich A. Effect of the Addition of Polyacrylic Acid of Different Molecular Weights to Coagulation Bath on the Structure and Performance of Polysulfone Ultrafiltration Membranes // Polymers 2023, 15, 1664. https://doi.org/10.3390/polym15071664
- Dmitrenko M., Sushkova X., Chepeleva A., Liamin V., Mikhailovskaya O., Kuzminova A., Semenov K., Ermakov S., Penkova A. Modification Approaches of Polyphenylene Oxide Membranes to Enhance Nanofiltration Performance // Membranes 2023, 13, 534. https://doi.org/10.3390/membranes13050534
- Prasad S., Penkova A., Chakroborty S., Praveen P. L. Fine Band Gap Tuning of Novel Azoxy Mesogens Versus Non-mesogen Molecules: Comparative Spectroscopic Analysis for Industrial Applications // Journal of Inorganic and Organometallic Polymers and Materials, 2023. https://doi.org/10.1007/s10904-023-02714-9
- Zhou J., Duan Y., Wu J., Penkova A., Huang R., Qi W., Su R. Spray-Drying Hydrophobic Cellulose Nanocrystal Coatings with Degradable Biocide Release for Marine Antifouling // Langmuir 2023, 39, 20, 7212–7220. https://doi.org/10.1021/acs.langmuir.3c00841
- Kuzminova A, Dmitrenko M, Salomatin K, Vezo O, Kirichenko S, Egorov S, Bezrukova M, Karyakina A, Eremin A, Popova E, et al. Holmium-Containing Metal-Organic Frameworks as Modifiers for PEBA-Based Membranes // Polymers. 2023, 15, 18, 3834. https://doi.org/10.3390/polym15183834
- Dmitrenko M., Kuzminova A., Cherian R.M., Joshy K.S., Pasquini D., John M.J., Hato M.J., Thomas S., Penkova A., Edible Carrageenan Films Reinforced with Starch and Nanocellulose: Development and Characterization // Sustainability. 2023, 15, 15817. https://doi.org/10.3390/su152215817
2022
- Dmitrenko Maria, Kuzminova Anna, Zolotarev Andrey, Markelov Denis, Komolkin Andrey, Loginova Evgenia, Plisko Tatiana, Burts K., Bildyukevich Alexandr, Penkova Anastasia. Modification strategies of polyacrylonitrile ultrafiltration membrane using TiO2 for enhanced antifouling performance in water treatment, Separation and Purification Technology, 286, 120500 (2022). DOI 10.1016/j.seppur.2022.120500.
- Dmitrenko Mariia, Chepeleva Anastasia, Liamin Vladislav, Mazur Anton, Semenov Konstantin, Solovyev Nikolay, Penkova Anastasia. Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol, Polymers, V. 14 (4), 691 (2022). DOI 10.3390/polym14040691.
- Dmitrenko Mariia, Atta Ramadan, Zolotarev Andrey, Kuzminova Anna, Ermakov Sergey, Penkova Anastasia. Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol. Sustainability. 14(6), 3561, (2022). https://doi.org/10.3390/su14063561
- Lakshmy K.S., Lal D., Nair A., Babu A., Das H., Govind N., Dmitrenko Mariia, Kuzminova Anna, Korniak Aleksandra, Penkova Anastasia, Tharayil A., Thomas S. Pervaporation as a Successful Tool in the Treatment of Industrial Liquid Mixtures, Polymers, 14, 8, 1604 (2022). doi: 10.3390/polym14081604
- Burts K., Plisko Tatiana, Dmitrenko Mariia, Zolotarev Andrey, Kuzminova Anna, Bildyukevich Alexandr, Ermakov Sergey, Penkova Anastasia. Novel Thin Film Nanocomposite Membranes Based on Chitosan Succinate Modified with Fe-BTC for Enhanced Pervaporation Dehydration of Isopropanol. Membranes 2022, 12, 653. https://doi.org/10.3390/membranes12070653
- Dmitrenko M., Chepeleva A., Liamin V., Kuzminova A., Mazur A., Semenov K., Penkova A. Novel PDMS-b-PPO Membranes Modified with Graphene Oxide for Efficient Pervaporation Ethanol Dehydration. Membranes (2022), 12, 832. https://doi.org/10.3390/membranes12090832
- Dmitrenko M.E., Kuzminova A.I., Zolotarev A.A., Korniak A.S., Ermakov S.S., Su R., Penkova A.V., Novel mixed matrix membranes based on polyelectrolyte complex modified with fullerene derivatives for enhanced pervaporation and nanofiltration. Separation and Purification Technology 2022, 298, 121649. 10.1016/j.seppur.2022.121649
- Plisko T., Burts K., Zolotarev A., Bildyukevich A., Dmitrenko M., Kuzminova A., Ermakov S., Penkova A., Development and Investigation of Hierarchically Structured Thin-Film Nanocomposite Membranes from Polyamide/Chitosan Succinate Embedded with a Metal-Organic Framework (Fe-BTC) for Pervaporation. Membranes 2022, 12, 967. https://doi.org/10.3390/membranes12100967
- Kuzminova A., Dmitrenko M., Zolotarev A., Myznikov D., Selyutin A., Su R., Penkova A. Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes 2022, 12, 908. https://doi.org/10.3390/membranes12100908
- Chen Shaohuang, Yue Ning, Cui Mei, Penkova Anastasia, Huang Renliang, Qi Wei, He Zhimin, Su Rongxin, Integrating direct reuse and extraction recovery of TEMPO for production of cellulose nanofibrils. Carbohydrate Polymers 2022, 29415, 119803. 10.1016/j.carbpol.2022.119803
- Jacob, J., Penkova, A.V. & Thomas, S. Special issue: emerging materials in nanofiltration membranes. emergent mater. 5, 1261–1262 (2022). https://doi.org/10.1007/s42247-022-00412-9
- Qiao Aihua, Huang Renliang, Penkova Anastasia, Qi Wei, He Zhimin, Su Rongxin, Superhydrophobic, elastic and anisotropic cellulose nanofiber aerogels for highly effective oil/water separation. Separation and Purification Technology 2022, 295, 121266. 10.1016/j.seppur.2022.121266
- Lin Zhongxin, Huang Renliang, Wu Jiangjiexing, Penkova Anastasia, Qi Wei, He Zhimin, Su Rongxin, Injectable self-healing nanocellulose hydrogels crosslinked by aluminum: Cellulose nanocrystals vs. cellulose nanofibrils, Chinese Journal of Chemical Engineering, 2022. 10.1016/j.cjche.2022.04.026
2021
- Penkova Anastasia V., Kuzminova Anna I., Dmitrenko Mariia E., Surkova Victoria A., Liamin Vladislav P., Markelov Denis A., Komolkin Andrei V., Poloneeva Daria Y., Laptenkova Anastasia V., Selyutin Artem A., Mazur Anton S., Emeline Alexei V., Thomas Sabu, Ermakov Sergey S. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66(NH2)-EDTA for highly efficient methanol isolation // Separation and Purification Technology, 263, 118370 (2021). doi: 10.1016/j.seppur.2021.118370. Impact factor: 5.774 (Q1)
- Dmitrenko Mariia, Liamin Vladislav, Lahderanta Erkki, Ermakov Sergey, Penkova Anastasia, Mixed matrix membranes based on sodium alginate modified by fullerene derivatives with L-amino acids for pervaporation isopropanol dehydration // Journal of Materials Science, 56, 12, 7765-7787 (2021). doi:10.1007/s10853-021-05791-8 56. Impact factor: 3.553 (Q2)
- Dmitrenko Mariia, Zolotarev Andrey, Liamin Vladislav, Kuzminova Anna, Mazur Anton, Semenov Konstantin, Ermakov Sergey, Penkova Anastasia, Novel Membranes Based on Hydroxyethyl Cellulose/Sodium Alginate for Pervaporation Dehydration of Isopropanol // Polymers, 13, 674 (2021). doi: 10.3390/polym13050674. Impact factor: 3.426 (Q1)
- Kuzminova Anna I., Dmitrenko Mariia E., Poloneeva Daria Y., Selyutin Artem A., Mazur Anton S., Emeline Alexei V., Mikhailovskii Vladimir Y., Solovyev Nikolay D., Ermakov Sergey S.,.Penkova Anastasia V, Sustainable composite pervaporation membranes based on sodium alginate modified by metal organic frameworks for dehydration of isopropanol // Journal of Membrane Science, 626, 119194 (2021). doi: 10.1016/j.memsci.2021.119194. Impact factor: 7.183 (Q1)
- Dmitrenko Mariia, Liamin Vladislav, Kuzminova Anna, Lahderanta Erkki, Solovyev Nikolay, Penkova Anastasia, Modification Approaches to Enhance Dehydration Properties of Sodium Alginate-Based Pervaporation Membranes // Membranes, 11, 255 (2021). doi:10.3390/membranes11040255. Impact factor: 3.094 (Q2)
- Kuzminova Anna, Dmitrenko Mariia, Mazur Anton, Ermakov Sergey, Penkova Anastasia, Novel Pervaporation Membranes Based on Biopolymer Sodium Alginate Modified by FeBTC for Isopropanol Dehydration // Sustainability, 13(11), 6092 (2021). doi: 10.3390/su13116092. Impact factor: 2.576 (Q2).
- Dmitrenko Mariia, Kuzminova Anna, Zolotarev Andrey, Liamin Vladislav, Plisko Tatiana, Burts Katsiaryna, Bildyukevich Alexandr, Ermakov Sergey, Penkova Anastasia, Novel High Flux Poly(m-phenylene isophtalamide)/TiO2 Membranes for Ultrafiltration with Enhanced Antifouling Performance // Polymers, 13, 2804 (2021). doi:10.3390/polym13162804. Impact factor: 3.426 (Q1).
- Dmitrenko Mariia, Kuzminova Anna, Zolotarev Andrey, Liamin Vladislav, Markelov Denis, Semenov Konstantin, Plisko Tatiana, Bildyukevich Alexandr, Penkova Anastasia. Novel pervaporation membranes based on hydroxyethyl cellulose/polyvinyl alcohol modified with fullerene derivatives for enhanced isopropanol dehydration. Journal of Material Research, V. 36 (24), p. 4986-5001 (2021). DOI 10.1557/s43578-021-00432-x.
- Sheveleva Nadezhda, Bezrodnyi Valeriy, Mikhtaniuk Sofia, Shavykin Oleg, Neelov Igor, Tarasenko I.I., Vovk M.A., Mikhailova M.E., Penkova Anastasia, Markelov Denis. Local Orientational Mobility of Collapsed Dendrimers, Macromolecules, V. 54 (23), p. 11083 – 1109214, 2021. DOI 10.1021/acs.macromol.1c01835.
2020
- Katsiaryna S. Burts, Tatiana V. Plisko, Alexandr V. Bildyukevich, Anastasia V. Penkova & Svetlana A. Pratsenko. Modification of polysulfone ultrafiltration membranes using block copolymer Pluronic F127. // Polymer Bulletin (2020), 1-28. doi: 10.1007/s00289-020-03437-4.
- Plisko, T. V., Bildyukevich, A. V., Burts, K. S., Hliavitskaya, T. A., Penkova, A. V., Ermakov, S. S. & Ulbricht, M., Modification of polysulfone ultrafiltration membranes via addition of anionic polyelectrolyte based on acrylamide and sodium acrylate to the coagulation bath to improve antifouling performance in water treatment. // Membranes (2020), 10 (10), 264; doi: 10.3390/membranes10100264
- Sofia E. Mikhtaniuk, Valeriy V. Bezrodnyi, Oleg V. Shavykin, Igor M. Neelov, Nadezhda N. Sheveleva, Anastasia V. Penkova, Denis A. Markelov. Comparison of Structure and Local Dynamics of Two Peptide Dendrimers with the Same Backbone but with Different Side Groups in Their Spacers. // Polymers (2020), 12(8), 1657; doi: 10.3390/polym12081657.
- Dmitrenko M., Zolotarev A., Plisko T., Burts K., Liamin V., Bildyukevich A., Ermakov S., Penkova A., Effect of the Formation of Ultrathin Selective Layers on the Structure and Performance of Thin-Film Composite Chitosan/PAN Membranes for Pervaporation Dehydration // Membranes (2020), 10, 153. doi: 10.3390/membranes10070153.
- Plisko T.V., Bildyukevich A.V., Burts K. S., Ermakov S.S., Penkova A.V., Kuzminova A.I., Dmitrenko M.E., Hliavitskaya T.A. and Ulbricht M., One-Step Preparation of Antifouling Polysulfone Ultrafiltration Membranes via Modification by a Cationic Polyelectrolyte Based on Polyacrylamide // Polymers (2020), 12, 1017. doi: 10.3390/polym12051017
- Mariia Dmitrenko, Vladislav Liamin, Anna Kuzminova, Anton Mazur, Erkki Lahderanta, Sergey Ermakov, Anastasia Penkova, Novel mixed matrix sodium alginate–fullerenol membranes: development, characterization, and study in pervaporation dehydration of isopropanol. // Polymers (2020), V.12, 864. doi: 10.3390/polym12040864
- M. Dmitrenko, A. Kuzminova, A. Zolotarev, S. Ermakov, D. Roizard, A. Penkova, Enhanced pervaporation properties of PVA-based membranes modified with polyelectrolytes. application to IPA dehydration. // Polymers (2020), 12. doi: 10.3390/polym12010014
- Pochkaeva E.I., Podolsky N.E., Zakusilo D.N., Petrov A.V., Charykov N.A., Vlasov T.D., Penkova A.V., Vasina L.V., Murin I.V., Sharoyko V.V., Semenov K.N., Fullerene derivatives with amino acids, peptides and proteins: From synthesis to biomedical application. // Progress in Solid State Chemistry (2020), V. 57, 100255. doi: 10.1016/j.progsolidstchem.2019.100255
2019
- Dmitrenko M.E., Penkova A.V., Kuzminova A.I., Atta R.R., Zolotarev A.A., Mazur A.S., Vezo O.S., Lahderanta E., Markelov D.A., Ermakov S.S., Development and investigation of novel polyphenylene isophthalamide pervaporation membranes modified with various fullerene derivatives. // Separation and Purification Technology (2019), V. 226, P. 241-251. doi: 10.1016/j.seppur.2019.05.092
- T.V. Plisko, A.V. Penkova, K.S. Burts, A.V. Bildyukevich, M.E. Dmitrenko, G.B. Melnikova, R.R. Atta, A.S. Mazur, A.A. Zolotarev, A.B. Missyul, Effect of Pluronic F127 on porous and dense membrane structure formation via non-solvent induced and evaporation induced phase separation. // Journal of Membrane Science (2019), V. 580, p. 336–349. doi: 10.1016/j.memsci.2019.03.028
- Benzaqui M., Semino R., Carn F., Tavares S.R., Menguy N., Giménez-Marques M., Bellido E., Horcajada P., Berthelot T., Kuzminova A.I., Dmitrenko M.E., Penkova A.V., Roizard D., Serre C., Maurin G., Steunou N., Covalent and Selective Grafting of Polyethylene Glycol Brushes at the Surface of ZIF‑8 for the Processing of Membranes for Pervaporation. // ACS Sustainable Chemistry & Engineering (2019), V. 7, I. 7, P. 6629-6639. doi: 10.1021/acssuschemeng.8b05587
- Ksenia Otvagina, Anastasia Penkova, Maria Dmitrenko, Anna Kuzminova, Tatyana Sazanova, Andrey Vorotyntsev, Ilya Vorotyntsev. Novel composite membranes based on chitosan copolymers with polyacrylonitrile and polystyrene: physicochemical properties and application for pervaporation dehydration of tetrahydrofuran // Membranes (2019), 9, 38. doi: 10.3390/membranes9030038.
- M.E.Dmitrenko, A.V.Penkova, R.R.Atta, A.A. Zolotarev, T.V.Plisko, A.S.Mazur, N.D.Solovyev, S.S.Ermakov. The development and study of novel membrane materials based on polyphenylene isophthalamide - Pluronic F127 composite // Materials & Design (2019), V. 165, 107596. doi: 10.1016/j.matdes.2019.107596
2018
- Bildyukevich A.V., Plisko T.V., Liubimova A.S., Penkova A.V., Dmitrenko M.E., Fullerenol-polyamide thin film composite hollow fiber membranes for low molecular weight cut-off ultrafiltration (2018), V. 62, No 2, P. 7-12.
- Dmitrenko M., Penkova A., Kuzminova A., Missyul A., Ermakov S., Roizard D., Development and Characterization of New Pervaporation PVA Membranes for the Dehydration Using Bulk and Surface Modifications. // Polymers (2018), 10, 571. doi: 10.3390/polym10060571
- Dmitrenko M.E., Penkova A.V., Kuzminova A.I., Morshed M., Larionov M.I., Alem H., Zolotarev A.A., Ermakov S.S., Roizard D. Investigation of new modification strategies for PVA membranes to improve their dehydration properties by pervaporation. // Applied Surface Science (2018), V. 450, P. 527-537. doi: 10.1016/j.apsusc.2018.04.169
- Penkova A.V., Dmitrenko M.E., Savon N.A., Missyul A.B., Mazur A.S., Kuzminova A.I., Zolotarev A.A., Mikhailovskii V., Lahderanta E., Markelov D.A., Semenov K.N., Ermakov S.S., Novel mixed-matrix membranes based on polyvinyl alcohol modified by carboxyfullerenes for pervaporation dehydration. // Separation and Purification Technology (2018), V. 204, P. 1–12. doi: 10.1016/j.seppur.2018.04.052
- Plisko T.V., Liubimova A.S., Bildyukevich A.V., Penkova A.V., Dmitrenko M.E., Mikhailovskii V.Y., Melnikova G.B., Semenov K.N., Doroshkevich N.V., Kuzminova A.I., Fabrication and characterization of polyamide-fullerenol thin film nanocomposite hollow fiber membranes with enhanced antifouling performance. // Journal of Membrane Science (2018), V. 551, P. 20–36. doi: 10.1016/j.memsci.2018.01.015
2017
- Matveev V.V., Markelov D.A., Dvinskikh S.V., Shishkin A.N., Tyutyukin K.V., Penkova A.V., Tatarinova E.A., Ignat'eva G.M., Milenin S.A., Investigation of Melts of Polybutylcarbosilane Dendrimers by 1H NMR Spectroscopy. // Scientific Reports (2017), Scientific Reports 7, Article number: 13710. doi: 10.1038/s41598-017-13743-z
- Semenov K.N., Andrusenko E.V., Charykov N.A., Litasova E.V., Panova G.G., Penkova A.V., Murin I.V., Piotrovskiy L.B., Carboxylated fullerenes: Physico-chemical properties and potential applications. // Progress in Solid State Chemistry (2017), V. 47–48, P. 19–36. doi: 10.1016/j.progsolidstchem.2017.09.001
- Dmitrenko M.E., Penkova A.V., Kuzminova A.I., Ermakov S.S., Roizard D., Investigation of polymer membranes modified by fullerenol for dehydration of organic mixtures. // IOP Conf. Series: Journal of Physics: Conf. Series (2017), 879 012010.
- Dmitrenko M.E., Penkova A.V., Missyul A.B., Kuzminova A.I., Markelov D.A., Ermakov S.S., Roizard D., Development and investigation of mixed-matrix PVA-fullerenol membranes for acetic acid dehydration by pervaporation. // Separation and Purification Technology (2017), V. 187, P. 285–293. doi: 10.1016/j.seppur.2017.06.061
- Penkova A.V.., Acquah S.F., Piotrovsly L.B., Markelov D.A., Semisalova A.S., Kroto H.W., Fullerene derivatives as nanoadditives for polymer composites. // Russian Chemical Reviews (2017), 86 (6), p. 530–566. doi: 10.1070/RCR4712
- Penkova A.V., Dmitrenko M. E., Ermakov S.S., Toikka A.M., Roizard D., Novel green PVA-fullerenol mixed matrix supported membranes for separating water-THF mixtures by pervaporation. // Environmental Science and Pollution Research (2017), P. 1–9. doi: 10.1007/s11356-017-9063-9
- Acquah S.F.A, Penkova A.V., Markelov D.A., Semisalova A.S., Leonhardt B.E., Magi J.M., Review - The Beautiful Molecule: 30 Years of C60 and its Derivatives. // ECS Journal of Solid State Science and Technology (2017), V. 6 (6), M1-M8. doi: 10.1149/2.0271706jss
2016
- Markelov D.A., Shishkin A.N., Matveev V.V., Penkova A.V., Lähderanta E., Chizhik V.I., Orientational Mobility in Dendrimer Melts: Molecular Dynamics Simulations. // Macromolecules (2016), V. 49, P. 9247−9257. doi: 10.1021/acs.macromol.6b01502
- Plisko T.V., Silaeva I.V., Penkova A.V., Bildyukevich A.V., Preparation, structure and properties of ultrafiltration membranes based on polyphenylene sulfone with additives of multi-walled carbon nanotubes. // Collection of scientific articles "Nanostructures in Condensed Matter" of the IX International Scientific Conference "Fullerenes and Nanostructures in Condensed Matter", ed. Vityaz P.A. and others, (2016), p. 257-264.
- Penkova A.V., Dmitrenko M.E., Sokolova M.P., Chen B., Plisko T.V., Markelov D.A., Ermakov S.S., Impact of fullerene loading on the structure and transport properties of polysulfone mixed-matrix membranes. // Journal of Materials Science (2016), 51(16), P. 7652-7659. doi: 10.1007/s10853-016-0047-9
- Markelov D.A., Matveev V.V., Ingman P., Nikolaeva M.N., Penkova A.V., Lahderanta E., Boiko N.I., Chizhik V.I., Unexpected Temperature Behavior of Polyethylene Glycol Spacers in Copolymer Dendrimers in Chloroform. // Scientific Reports (2016), | 6:24270 |. doi: 10.1038/srep24270
- Penkova A.V., Acquah S.F.A., Dmitrenko M.E., Sokolova M.P., Mikhailova M.Е., Polyakov E.S., Ermakov S.S., Markelov D.A., Roizard D., Improvement of pervaporation PVA membranes by the controlled incorporation of fullerenol nanoparticles. // Materials & Design (2016), V. 96, P. 416–423. doi: 10.1016/j.matdes.2016.02.046
- Penkova A.V., Acquah S.F.A., Dmitrenko M.E., Sokolova M.P., Mikhailova M.Е., Polyakov E.S., Ermakov S.S., Markelov D.A., Roizard D., Improvement of pervaporation PVA membranes by the controlled incorporation of fullerenol nanoparticles. // Materials & Design (2016), V. 96, P. 416–423. doi: 10.1016/j.matdes.2016.02.046
- Shishov A., Penkova A., Zabrodin A., Nikolaev K., Dmitrenko M., Ermakov S., Bulatov A., Vapor permeation-stepwise injection simultaneous determination of methanol and ethanol in biodiesel with voltammetric detection. // Talanta (2016), V. 148, P. 666–672. doi: 10.1016/j.talanta.2015.05.041
2015
- Toikka A., Naumkin P., Penkova A., Approximation and analysis of pervaporation of binary mixtures using nonequilibrium thermodynamics approach. // Chemical Engineering Research and Design (2015), V. 104, P. 669–680. doi: 10.1016/j.cherd.2015.10.007
- Penkova A.V., Acquah S.F.A., Sokolova M.P., Dmitrenko M.E., Toikka A.M., Polyvinyl alcohol membranes modified by low-hydroxylated fullerenol C60(OH)12. // Journal of Membrane Science (2015), V.49, P. 122–27. doi: 10.1016/j.memsci.2015.05.011
- Penkova A., Polotskaya G., Toikka A., Pervaporation Сomposite Membranes for Ethyl Acetate Production. // Chemical Engineering and Processing: Process Intensification (2015), V. 87, P. 81–87. doi: 10.1016/j.cep.2014.11.015
2014
- Toikka A.M, Penkova A.V., Markelov D.A., Description and approximation of mass-transfer in pervaporation process on the base of nonequilibrium thermodynamics approach. // International Journal of Heat and Mass Transfer (2014), V.72, P. 423–429. doi: 10.1016/j.ijheatmasstransfer.2014.01.027
- Penkova A. V., Acquah S. F. A., Dmitrenko M. E., Chen B., Semenov K. N., Kroto Harold W., Transport Properties of Cross-Linked Fullerenol-PVA Membranes. // Carbon (2014), V. 76, P. 446 –450. doi: 10.1016/j.carbon.2014.04.053
2004–2013
- Penkova A.V., Polotskaya G.A.,Toikka A.M. Separation of acetic acid–methanol–methyl acetate–water reactive mixture. // Chemical Engineering Science (2013), V.101, Pages 586–592. doi: 10.1016/j.ces.2013.05.055
- Sudareva N.N., Penkova A.V., Kostereva T.A., Polotskii A.E., Polotskaya G.A., Properties of casting solutions and ultrafiltration membranes based on fullerene-polyamide nanocomposites. // eXPRESS Polymer Letters (2012), V. 6, No.3, P. 178–188A. doi: 10.3144/expresspolymlett.2012.20
- Penkova A.V., Pientka Z., Polotskaya G.A., MWCNT/poly(phenylene-iso-phtalamide) Nanocomposite Membranes for Pervaporation of Organic Mixture. // Fullerenes, Nanotubes and Carbon Nanostructures (2011), V. 19, P. 137-140. doi: 10.1080/1536383X.2010.490138
- Penkova A.V., Polotskaya G.A., Toikka A.M., Kocherbitov V.V. Effect of Residual Solvent on Physicochemical Properties of Poly(Phenylene Isophtalamide) Membrane. // Drying Technology (2011), V. 29, P. 633–641.
- Penkova A.V., Polotskaya G.A., Gavrilova V.A., Toikka A.M., J.-C. Liu, Trchova M., Slouf M., Pientka Z. Polyamide Membranes Modified by Carbon Nanotubes: Application for Pervaporation. // Separation Science and Technology (2010), V. 45, p. 35–41. doi: 10.1080/01496390903401812
- Polotskaya G.A., Penkova A.V., Pientka Z., Toikka A.M., Polymer membranes modified by fullerene C60 for pervaporation of organic mixtures. // Desalination and Water Treatment (2010), V. 14. p. 83–88. doi: 10.5004/dwt.2010.1528
- Penkova A.V., Markelov D.A., Toikka A.M. Thermodynamic modeling of the evaporation process of binary solutions through a membrane. // Vestniks of Saint Petersburg University (2010), 4, 3, p. 68-76.
- Penkova A.V., Polotskaya G.A., Toikka A.M., Trchova M., Slouf M., Urbanova M., Brus J., Brozova L., Pientka Z., Structure and Pervaporation Properties of Poly(phenylene-iso-phtalamide) Membranes Modified by Fullerene C60. // Macromolecular Materials and Engineering (2009), V. 294, p. 432-440. doi: 10.1002/mame.200800362
- Penkova A., Toikka A., Kostereva T., Sudareva N., Polotskaya G., Structure and transport properties of fullerene – polyamide membranes. // Fullerenes, Nanotubes, and Carbon Nanostructures (2008), V. 16, No. 5-6, p. 666-669. doi: 10.1080/15363830802314251
- Polotskaya G.A., Pen'kova A.V., Sudareva N.N., Polotskii A.E., Toikka A.M., Polyamide ultrafiltration membranes modified with nanocarbon additives. // Russian Journal of Applied Chemistry (2008), V.81, No. 2, p. 236-240.
- Polotskaya G.A., Penkova A.V., Toikka A.M., Pientka Z., Brozova L., Bleha M., Transport of small molecules through polyphenylene oxide membranes modified by fullerene. // Separation Science and Technology (2007), V. 42, No. 2, p. 333-347. doi: 10.1080/01496390600997963
- Polotskaya G.A., Penkova A.V, Toikka A.M., Fullerene-containing polyphenylene oxide membranes for pervaporation. // Desalination (2006), V. 200, No. 1-3, p. 400-402. doi: 10.1016/j.desal.2006.03.347
- Polotskaya G.A., Gladchenko S.V., Penkova A.V., Kuznetsov V.M., Toikka A.M., Membranes based on fullerene-modified polyphenylene oxide for separation of aqueous-organic mixtures. // Russian Journal of Applied Chemistry (2005), V. 78, No 9, p. 1493-1498.
- Kuznetsov U.P., Khripunov A.K., Kruchinina E.V., Turkova A.D., Penkova A.V., Transport properties of membranes based on cellulose myristinate in the separation of mixtures of gases or liquids. // Russian Journal of Applied Chemistry (2004), V. 77, No 11, p. 1895-1900.
Patents
- Polotskaya G.A., Penkova A.V. A method of obtaining composite membranes with a fullerene-containing polymer selective layer. Patent No. RU2414953. Publication date. 27.03.2011. Application No. 2009127219/04. Priority date 14.07.2009.
- Polotskaya G.A., Penkova A.V. Installation for the preparation of composite polymer membranes. Patent No. 88009. BI No. 30, 2009 year. Publication date. 27.10.2009. Application No. 2009129016. Priority date 27.07.2009.
- Penkova A.V., Semenov K.N. A method of obtaining diffusion fullerenol-containing membranes. Priority date 15.07.2012. Application No. 2012127842. Patent No. RU2501597. Publication date 20.12.2013.
- Penkova A.V. Device for producing diffusion polymer membranes. Priority date 05.10.2012. Application No. 2012142277. Patent No. RU2504429. Publication date. 20.01.2014.
- Kuzminova A.I., Penkova A.V. The device for producing a composite membrane with polyelectrolyte layers. Application No. 2020122996. Patent No. RU 2759899 C1. Registration date 06.07.2020. Publication date 18.11.2021.
Science Projects
Grants management by Penkova Anastasia
- 2010 — grant for participation in NanoMemCourse EA3: Nano-structured materials and Membranes in the Food Industry (Cetraro and Rende, Calabria, Italy).
- 2010 — grant of the Government of St. Petersburg for young scientists to carry out scientific research in the field of membrane processes.
- 2011 — grant of Russian Foundation for Basic Research No. 09-03-09371 «Mobility of young scientists to participate in the international conference «Euromembranes 2009».
- 2011 — grant of Russian Foundation for Basic Research No. 11-03-09471 «Presentation of an invited oral talk at the international conference ICM-2011, dedicated to membrane methods for cleaning the environment and biological objects».
- 2010–2013 — grant of Bortnik Fund "U.M.N.I.K." of “Participant in the youth scientific and innovation competition”.
- 2011–2013 — grant of Ministry of Education and Science of Russian Federation “Scientific and scientific-pedagogical personnel of innovative Russia” for 2009–2013. “Conducting scientific research by young PhD candidates of science in the following areas:
- nanotechnology and nanomaterials;
- mechatronics and the creation of microsystem technology;
- the creation of biocompatible materials;
- creation and processing of composite and ceramic materials;
- creation and processing of crystalline materials;
- creation and processing of polymers and elastomers;
- the creation of membranes and catalytic systems;
- metallurgical technologies;
- construction technologies. ” Application code: 2011-1.3.1-207-008-058 GK 16.740.11.0658 dated 02.06.2011.
- 2011 — SPbSU project «Internship of A.V. Penkova in the group of Nobel laureate Harold Walter Kroto, at the Florida State University (USA), Department of Chemistry and Biochemistry in order to master the methods of research of composite materials».
- 2012 — grant of the Government of St. Petersburg for young scientists and PhD for research in the field of membrane processes.
- 2012 — grant of Russian Foundation for Basic Research No. 12-08-90823-mol_rf_nr “Determination of the effect of fullerene additives on the gas separation properties of polysulfone-based membranes”. Scientific project of Penkova Anastasia from St. Petersburg State University, St. Petersburg, in the Nizhny Novgorod State Technical University of R.E. Alekseeva, Nizhny Novgorod.
- 2012–2013 — grant of the Russian Science Foundation No. 12-03-33155 mol_ved “Novel materials based on polymers and polymer nanocomposites. The study of dynamic and equilibrium properties: theory, computer modeling, experiment”.
- 2013 — grant of Russian Foundation for Basic Research No. 13-08-90713 mol_rf_nr “Determination of the effect of surface morphology of nanocomposite polymer membranes on their physicochemical and transport characteristics”. Scientific project of Penkova Anastasia from St. Petersburg State University, St. Petersburg, in the Nizhny Novgorod State Technical University of R.E. Alekseeva, Nizhny Novgorod.
- 2014 — grant of the Government of St. Petersburg for young scientists, young candidates of sciences of universities, industry and academic institutions to conduct research in the field of membrane processes.
- 2015–2017 — grant of Russian Foundation for Basic Research No. 15-58-04034 bel_mol_а. “New hybrid polymer materials for baromembrane and diffusion separation processes: production, structure and properties”.
- 2015–2016 — President's scholarship No. СП-1153.2015.1 «Development of energy-saving technologies for obtaining high-purity substances using new membrane materials».
- Grant of the Government of St. Petersburg for young scientists, young PhD of universities, industrial and academic institutes «Synthesis of membranes with improved physicochemical and transport characteristics for the separation of industrially significant mixtures in the process of pervaporation».
- 2016 — grant of Russian Foundation for Basic Research No. 16-38-50146 mol_nr “Preparation and characterization of dense mixed matrix membranes”.
- 2016 — grant of the Government of St. Petersburg for young scientists and PhD “Development and study of novel supported membranes for the separation of industrially significant mixtures during pervaporation”.
- 2017 — grant of the Russian Science Foundation of Initiative research by young scientists of the Presidential Research Projects Program No. 17-73-20060 “Development of new mixed matrix membranes for highly efficient, environmentally friendly and resource-saving separation of liquid mixtures”.
- 2018 — grant of Russian Foundation for Basic Research No. 17-38-50087 mol_nr “Study of the transport properties of new supported pervaporation membranes with a selective layer based on chitosan in the process of tetrahydrofuran dehydration”.
- 2019 — grant of Russian Foundation for Basic Research No. 17-58-04067 bel_mol_a “Novel membrane materials for dehydration and water treatment”.
- 2019–2021 — grant of Russian Foundation for Basic Research No. 19-38-90008 Postgraduate students «Development and study of new membranes based on sodium alginate modified with organometallic framework polymers».
- 2020–2023 — grant of Russian Science Foundation Competition 2020 «Conducting research by scientific groups under the guidance of young scientists» of the President's program of research projects implemented by leading scientists, including young scientists No. 20-79-10064 «Development of new mixed matrix membranes based on cellulose derivatives for highly efficient, environmentally friendly and resource-saving membrane separation of liquid mixtures and creation of catalytic membrane reactors».
- 2020–2022 — grant of Russian Foundation for Basic Research No. 20-38-51022 Scientific mentoring «Creation of new composite membrane-catalytic systems for power plants and research of their catalytic and membrane-separation properties in water treatment processes».
- 2022–2024 — grant of BRICS №075-15-2022-1231 on 18.10.2022« Sustainable green nanocomposite for smart edible packaging applications», funded by the Russian Federation represented by the Ministry of Science and Higher Education.
- 2023–2025 — grant of Russian Science Foundation Competition 2023 to extend the deadlines for projects supported by grants from the Russian Science Foundation for the event "Conducting research by scientific groups led by young scientists" of the Presidential Program of research projects implemented by leading scientists, including young scientists №. 20-79-10064-П "Development of new mixed matrix membranes based on cellulose derivatives for highly efficient, environmentally friendly and resource-saving membrane separation of liquid mixtures and the creation of catalytic membrane reactors".
Grants management by Dmitrenko Mariia
- 2014–2016 years: the Bortnik Fund "UMNIK" «Development and research of properties of new nanocomposite membranes based on polyvinyl alcohol»;
- 2014–2018 years: grants from St. Petersburg State University for an internship in the laboratory of the University of Lorraine, Nancy (France) under the guidance of the director of the membrane group Denis Roisard;
- 2019–2021 years: grant RSF No. 19-73-00105 «Development of novel mixed matrix membranes for the development of an environmentally friendly and resource-saving pervaporation process»;
- 2021 year: grant RSF No. 21-73-00043 «Development of novel mixed matrix membranes based on polyphenylene oxide and polydimethylsiloxane for the development of highly efficient and resource-saving membrane processes pervaporation and nanofiltration»;
- 2023 year: grant RSF №23-73-01070 « Development of novel mixed matrix membranes based on polyelectrolyte complexes for highly efficient and resource-saving membrane processes».
Grants management by Kuzminova Anna
- 2017 year: grant from St. Petersburg State University for an internship in the laboratory of the University of Lorraine, Nancy (France) under the guidance of the director of the membrane group Denis Roisard;
- 2018–2020 years: grant from the Foundation for Assistance to Innovation, winner of the UMNIK program «Development of novel pervaporation membranes for the separation of industrially significant mixtures»;
- 2023–2024 years — grant RSF №23-29-00473 «Novel highly efficient membrane materials based on polymer/metal-organic framework composites for water treatment in pervaporation and nanofiltration processes».
Information for Students
We invite students and postgraduates to complete diploma and dissertation works in the following areas:
- Development of new polymer membrane materials, including biomaterials, for diffusion membrane processes and the study of their physical and chemical characteristics by various methods of analysis;
- Development of new polymer membrane materials for baromembrane membrane processes, including biomaterials, and the study of their physical and chemical characteristics by various methods of analysis;
- Optimization of membrane processes in order to develop sustainable development processes.
Awards and Prizes
Awards and prizes of Penkova Anastasia
- Winner of the L’Oréal-UNESCO fellowship "For Women in Science" (2018).
- Laureate of the National Competition of Innovation Projects, 1st place in the rating "Top 100 Young Innovative Leaders of Russia", in the nomination "Chemical Industry". Diploma of the absolute winner (2011).
- Laureate of the Generation Foundation contest, award in the nomination “The Best Scientist in the Field of the Study of Nanomaterials and Nanotechnologies” (2011).
- Laureate of the XXIII competition of the European Academy for young scientists of Russia in the section "Chemistry" (2017).
- Prize of the St. Petersburg State University for scientific works “for contribution to the science of young researchers” (2017).
- Laureate of the VI All-Russian Internet Olympiad in Nanotechnology (intellectual forum “Nanotechnology - a breakthrough into the future!”), Winner diploma (2012).
- Diploma of the 4th All-Russian Internet Olympiad in nanotechnology "Nanotechnology - a breakthrough into the future" - the winner of the creative competition "Academic Approach".
- Scholarship of the President of the Russian Federation for young scientists (2015).
- Scholarship of the President of the Russian Federation (2009–2010).
- Winner diploma of the Government of St. Petersburg for young scientists without a degree (2010).
- A diploma of the winner of the competition of the Government of St. Petersburg for young scientists, the implementation of scientific research in the field of membrane processes (2012).
- Winner diploma of the competition of the Government of St. Petersburg for young scientists with a degree of candidate of sciences (2012).
- The diploma of the winner of the competition of the Government of St. Petersburg for young scientists, young candidates of science, universities, industry and academic institutions (2014).
- Winner diploma of the competition of the Government of St. Petersburg for young scientists with a Ph.D. (2015).
- Winner diploma of the competition of the Government of St. Petersburg for young scientists with a Ph.D. degree (2016).
- Prize of the Government of St. Petersburg in the field of scientific and pedagogical activity (2016).
- Prize of the Government of St. Petersburg in the field of scientific and pedagogical activity (2017).
- Diploma of the II international competition of scientific works of young scientists in the field of nanotechnology (2009).
- Diploma of the XVI International Conference "Lomonosov-2009" for the best report (2009).
- Diploma at the International Symposium "Fullerenes and Atomic Clusters" (IWFAC 2009) for the best report (2009).
- Laureate diploma for the best report at the 13th International Youth School-Conference "Magnetic resonance and its applications - Spinus-2016" (2016).
- 1st degree diploma for the presented work "Development of new membranes based on polyvinyl alcohol modified with an organometallic framework polymer UIO-66(NH2)-EDTA" in the nomination "Scientific articles in chemical sciences" in the 33rd International competition of scientific research works (28 February 2021).
- Vinner of "Golden Names of Higher School" in the nomination "For the development of international cooperation in the field of higher education" (2023).
Awards and prizes of Dmitrenko Mariia
- Diploma for the best poster at VI International Conference on Chemistry "Mendeleev—2012", Section 4 — Physical Chemistry, St. Petersburg (2012);
- Increased academic stipend of St. Petersburg State University (2013);
- Certificate of Merit for the first place in the competition for the Best Research Poster at Chemistry PhD Conference Institute of Chemistry St. Petersburg State University (2014);
- The winner in the nomination of a research project in a competition of business ideas, scientific and technical developments and research projects “Young, Daring, Perspectives” (2016) (the competition is held by the Committee on Science and High Education in accordance with the Decree of the Government of St. Petersburg from 30.06.2010 No. 883);
- The winner of the university award "For Scientific Works" in the category "For the contribution of young researchers to science " and for the series of works "Transport characteristics and physicochemical properties of polymeric membranes modified with carbon nanoparticles" (December 25, 2017);
- Diploma of laureate for poster presentations at 15 International Youth School-Conference “Magnetic resonance and its application. SPINUS” (April 1–6, 2018, Saint-Petersburg);
- The winner of subsidies to young scientists, young candidates of science universities, industry and academic institutions located in St. Petersburg (November 26, 2018, diploma of the series PSP No. 18800);
- Diploma of II degree for the best oral report among young scientists presented at the XIV Scientific Conference (with international participation) “Membranes-2019”, 21-25 October, Sochi (2019);
- Diploma of I degree for the presented work "Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Metal-Organic Framework UiO-66(NH2)-EDTA" in the nomination "Scientific articles in chemical sciences" in the 33rd International Research Competition (28.02.2021).
- 1st degree diploma for the presented work "Development of new membranes based on polyvinyl alcohol modified with an organometallic framework polymer UIO-66(NH2)-EDTA" in the nomination "Scientific articles in chemical sciences" in the 33rd International competition of scientific research works (28 February 2021).
Awards and prizes of Kuzminova Anna
- Laureate of the competition of the best poster presentation 16th International School-Conference «Spinus 2019».
- Diploma of the 1st degree for a report at the international scientific-practical conference "The predictive nature of scientific research and the practice of their implementation in the context of the global crisis in the economy and society".
- Winner of the competitive for a scholarship of the Government of the Russian Federation in the 2019–2020 academic year.
- 2020 year: Winner of the competition for grants for students of universities located in St. Petersburg, graduate students of universities, industry and academic institutions located in St. Petersburg.
- 2021 — the winner of the competition for grants for students of universities located on the territory of St. Petersburg, graduate students of universities, industry and academic institutions located on the territory of St. Petersburg.
- 1st degree diploma for the presented work "Development of new membranes based on polyvinyl alcohol modified with an organometallic framework polymer UIO-66(NH2)-EDTA" in the nomination "Scientific articles in chemical sciences" in the 33rd International competition of scientific research works (28 February 2021).
Department of Organic Chemistry
Rooms 3186 (office), 3193 (lab)
Noncovalent Organocatalysis
Short URL for media go.spbu.ru/rgbolotin
In the past decade, organocatalysis has been the focus of extensive studies owing to its significant advantages over catalysis by metal-containing species including lower toxicity, reduced environmental footprint, and low to negligible sensitivity to air and moisture. In general, organocatalysts can function either through a covalent or noncovalent bonding activation. A covalent activation mode involves the formation of covalent bond(s) between a substrate and catalyst, whereas a noncovalent mode involves the activation of substrates through noncovalent linkages to the catalyst. For noncovalent catalysis, an organic catalyst typically interacts with a substrate through hydrogen bonding, whereas catalytic reactions involving halogen or chalcogen bonding are far less explored.

Group Members
 |
Group leader Bolotin Dmitrii S.Full professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Il’in Mikhail V.Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Sysoeva Alexandra A.Junior researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Putnin Ivan O.Graduate Student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Polonnikov Denis A.Research assistant This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Safinskaya Yana V.Research assistant This email address is being protected from spambots. You need JavaScript enabled to view it. |
Research Interests
Study of noncovalent oranocatalysis is a complex task requiring complementary research in the following scientific fields:
 |
Synthetic organic and organoelement chemistry. Our group is focused on rational design of organoelement species exhibiting high catalytic activity in transformations of organic and coordination compounds. Compounds of our general interest are cationic heterocycles featuring exo- and endocyclic halogen or chalcogen atoms, as well as urea and squaramide derivatives. |
 |
Physical organic chemistry. Study of catalytic activity includes determination of kinetic and thermodynamic parameters of the studied model reactions. This research is performed using spectroscopic techniques (NMR, IR, UV-Vis) and thermal analyses. Determination of influence of electronic and steric factors on kinetic and thermodynamic parameters of the reactions allows us to conclude plausible mechanisms of reactions and mechanisms of the catalysis. |
 |
Theoretical calculations. Plausible mechanisms of catalysis are typically confirmed (or rejected) by us using DFT calculations. Theoretical calculations are also performed before the synthetic work to shed light on the most promising compounds, which should exhibit the highest catalytic activity. |
Current Grants
 Grant # 103922061 New Generation Organocatalysts as a Replacement for Metal-containing Lewis Acids for Chemical and Pharmaceutical Industries (Bolotin D.S.).
Grant # 103922061 New Generation Organocatalysts as a Replacement for Metal-containing Lewis Acids for Chemical and Pharmaceutical Industries (Bolotin D.S.).
 Grant #23-23-00091 New Catalytic Systems Based on Bifunctional Complexes of Late 3d-metals Featuring Halogen Bond-Donating Ligands (Il'in M.V.).
Grant #23-23-00091 New Catalytic Systems Based on Bifunctional Complexes of Late 3d-metals Featuring Halogen Bond-Donating Ligands (Il'in M.V.).
 Grant #23-73-10003 Organocatalysis Based on sigma-hole carriers for Transition to Ecological Energy- and Resource-saving Processes (Bolotin D.S.).
Grant #23-73-10003 Organocatalysis Based on sigma-hole carriers for Transition to Ecological Energy- and Resource-saving Processes (Bolotin D.S.).
Recent Publications
2024
Q1 Sysoeva A.A., Il’in M.V., Bolotin D.S., Cooperative Covalent–Noncovalent Organocatalysis of the Knoevenagel Condensation Based on an Amine and Iodonium Salt Mixture, ChemCatChem, 2024, in press. DOI: 10.1002/cctc.202301668.
 The experimentally obtained kinetic data has indicated the existence of a synergetic cocatalytic effect provided by an iodonium salt in the base-catalyzed Knoevenagel condensation. The diphenyliodonium triflate serving as the halogen bond-donating Lewis acid provides the higher cocatalytic effect than zinc(II) triflate or triflic acid serving, respectively, as the metal cation-based Lewis acid and Brønsted acid.
The experimentally obtained kinetic data has indicated the existence of a synergetic cocatalytic effect provided by an iodonium salt in the base-catalyzed Knoevenagel condensation. The diphenyliodonium triflate serving as the halogen bond-donating Lewis acid provides the higher cocatalytic effect than zinc(II) triflate or triflic acid serving, respectively, as the metal cation-based Lewis acid and Brønsted acid.
Such a cocatalytic effect remains the same for a broad scope of carbonyl compounds covering aldehydes featuring electron-withdrawing or electron-donating substituents, as well as ketones involved in the reaction.
Q1 Il’in M.V., Safinskaya Ya.V., Polonnikov D.A., Novikov A.S., Bolotin D.S., Chalcogen- and Halogen-Bond-Donating Cyanoborohydrides Provide Imine Hydrogenation, J. Org. Chem., 2024, in press. DOI: 10.1021/acs.joc.3c02282.
 Sulfonium, selenonium, telluronium, and iodonium cyanoborohydrides have been synthesized, isolated, and fully characterized by various methods, including single-crystal X-ray diffraction (XRD) analysis.
Sulfonium, selenonium, telluronium, and iodonium cyanoborohydrides have been synthesized, isolated, and fully characterized by various methods, including single-crystal X-ray diffraction (XRD) analysis.
The quantum theory of atoms in molecules’ analysis based on the XRD data indicated that the hydride···σ-hole short contacts observed in the crystal structures of each compound have a purely noncovalent nature. The telluronium and iodonium cyanoborohydrides provide a significantly higher rate of the model reaction of imine hydrogenation compared with sodium and tetrabutylammonium cyanoborohydrides. Based on the NMR and high-resolution electrospray ionization mass spectrometry data indicating that the reaction progress is accompanied by the cation reduction, a mechanism involving intermediate formation of elusive onium hydrides has been proposed as an alternative to conventional electrophilic activation of the imine moiety by its ligation to the cation’s σ-hole.
2023
Sysoeva A.A., Novikov A.S., Suslonov V.V., Bolotin D.S., Il'in M.V., 2,1,3-Benzoselenadiazole-containing zinc(II) halide complexes: Chalcogen bonding in the solid state and catalytic activity in the Schiff condensation, Inorg. Chim. Acta, 2024, 561, 121867. DOI: 10.1016/j.ica.2023.121867.
 XRD study of crystals of zinc(II) halide complexes featuring 2,1,3-benzoselenadiazole (L) ligands [ZnL2X2] (X=Cl, Br, I) indicated availability of intramolecular chalcogen bonds between the Se atom and the halide ligands, which affect a rotation barrier around the Zn–N bonds and thus control a geometry of these species. In the solid state, the Se atom additionally forms intermolecular chalcogen bonds with the N atom of the 2,1,3-benzoselenadiazole ligands, which results in formation of infinite 1D chains. Theoretical calculations and experimental kinetic study indicated that although the Se atom serves as a Lewis acid, coordination of the organic ligand to the zinc(II) center does not provide enhanced catalytic activity of the latter in the model reaction of the Schiff condensation.
XRD study of crystals of zinc(II) halide complexes featuring 2,1,3-benzoselenadiazole (L) ligands [ZnL2X2] (X=Cl, Br, I) indicated availability of intramolecular chalcogen bonds between the Se atom and the halide ligands, which affect a rotation barrier around the Zn–N bonds and thus control a geometry of these species. In the solid state, the Se atom additionally forms intermolecular chalcogen bonds with the N atom of the 2,1,3-benzoselenadiazole ligands, which results in formation of infinite 1D chains. Theoretical calculations and experimental kinetic study indicated that although the Se atom serves as a Lewis acid, coordination of the organic ligand to the zinc(II) center does not provide enhanced catalytic activity of the latter in the model reaction of the Schiff condensation.
Q1 Il’in M.V., Polonnikov D.A., Novikov A.S., Sysoeva A.A., Safinskaya Ya.V., Bolotin D.S., Influence of Coordination to Silver(I) Centers on the Activity of Heterocyclic Iodonium Salts Serving as Halogen-Bond-Donating Catalysts, ChemPlusChem, 2023, 88, e202300304. DOI: 10.1002/cplu.202300304.
 Kinetic data based on the 1H NMR monitoring and computational studies indicate that in a solution pyrazole-containing iodonium triflates and silver(I) triflate bind each other, and such an interplay results in the decrease of the total catalytic activity of the mixture of these Lewis acids compared to the separate catalysis of the Schiff condensation, the imine–isocyanide coupling, or the nucleophilic attack on a triple carbon–carbon bond. Moreover, the kinetic data indicate that such a cooperation with the silver(I) triflate results in prevention of decomposition of the iodonium salts during the reaction progress. XRD study confirms that the pyrazole-containing iodonium triflate coordinates to the silver(I) center via the pyrazole N atom to produce a rare example of a pentacoordinated trigonal bipyramidal dinuclear silver(I) complex featuring cationic ligands.
Kinetic data based on the 1H NMR monitoring and computational studies indicate that in a solution pyrazole-containing iodonium triflates and silver(I) triflate bind each other, and such an interplay results in the decrease of the total catalytic activity of the mixture of these Lewis acids compared to the separate catalysis of the Schiff condensation, the imine–isocyanide coupling, or the nucleophilic attack on a triple carbon–carbon bond. Moreover, the kinetic data indicate that such a cooperation with the silver(I) triflate results in prevention of decomposition of the iodonium salts during the reaction progress. XRD study confirms that the pyrazole-containing iodonium triflate coordinates to the silver(I) center via the pyrazole N atom to produce a rare example of a pentacoordinated trigonal bipyramidal dinuclear silver(I) complex featuring cationic ligands.
Q1 Sysoeva A.A., Novikov A.S., Il'in M.V., Bolotin D.S., Solvent-modulated binding selectivity of reaction substrates to onium-based sigma-hole donors, Catal. Sci. Technol., 2023, 13, 3375-3385. DOI: 10.1039/D3CY00071K.
 The combination of experimental data and results of DFT calculations indicates that the catalytic activity of chalconium and halonium salts serving as sigma-hole donating organocatalysts cannot be clearly estimated via analysis of the electrostatic potential on the catalysts’ sigma-holes and values of the catalyst•••TS intermolecular interactions, such as polarization effects, charge transfer, or covalency of bonding. Moreover, the real catalytic effect might not correlate well with the values of Gibbs free energy of activation of the reactions, because solvation effects and other competitive binding processes play at least an equal or even more important role in the catalysis. It was shown in the present work that the solvation can either lead to the increase of equilibrium concentration of reactive catalyst•••electrophile associates, thus accelerating the reaction, or brings favorable generation of catalyst•••nucleophile species resulting in the suppression of the catalytic activity of the organocatalyst.
The combination of experimental data and results of DFT calculations indicates that the catalytic activity of chalconium and halonium salts serving as sigma-hole donating organocatalysts cannot be clearly estimated via analysis of the electrostatic potential on the catalysts’ sigma-holes and values of the catalyst•••TS intermolecular interactions, such as polarization effects, charge transfer, or covalency of bonding. Moreover, the real catalytic effect might not correlate well with the values of Gibbs free energy of activation of the reactions, because solvation effects and other competitive binding processes play at least an equal or even more important role in the catalysis. It was shown in the present work that the solvation can either lead to the increase of equilibrium concentration of reactive catalyst•••electrophile associates, thus accelerating the reaction, or brings favorable generation of catalyst•••nucleophile species resulting in the suppression of the catalytic activity of the organocatalyst.
2022
Q1 Novikov A.S., Bolotin D.S., Хеnon Derivatives as Aerogen Bond-Donating Catalysts for Organic Transformations: A Theoretical Study on the Metaphorical "Spherical Cow in a Vacuum" Provides Insights into Noncovalent Organocatalysis, J. Org. Chem., 2023, 88, 1936–1944. DOI: 10.1021/acs.joc.2c00680
Computations indicate that cationic and non-charged xenon derivatives should exhibit higher catalytic activity than their iodine-based noncovalent organocatalytic congeners. Perfluorophenyl xenonium(II) is expected to demonstrate the best balance between catalytic activity and chemical stability for use in organocatalysis. Comparing its catalytic activity with that of isoelectronic perfluoroiodobenzene indicates that the high catalytic activity of cationic noncovalent organocatalysts is predominantly attributed to the electrostatic interactions with the reaction substrates, which cause the polarization of the ligated species during the reaction progress. In contrast, the electron transfer and covalent contributions to the bonding between the catalyst and substrate have negligible effects. The dominant effect of the electrostatic interactions results in a strong negative correlation between the calculated Gibbs free energies of activation for the modeled reactions and the highest potentials of the σ-holes on the central atoms of the catalysts. No such correlation is observed for the non-charged catalysts.
Q1 Polonnikov D.A., Il'in M.V., Safinskay Ya.V., Aliyarova I.S., Novikov A.S., Bolotin D.S., (Pre)association as a Crucial Step for Computational Prediction and Analysis of the Catalytic Activity of Sigma-Hole Donating Organocatalysts, Org. Chem. Front., 2023, 10, 169–180. DOI: 10.1039/D2QO01648F
 Based upon the experimentally obtained kinetic data on iodonium salt catalyzed nucleophilic addition of isocyanide to imine leading to imidazopyridine species, a reliable model for DFT calculations has been suggested. It was shown that preassociation of the catalysts with the reaction species might significantly affect the total energy profile of the reaction obtained by DFT. The associates of the organocatalysts featuring the solvent molecules ligated to its sigma-holes should be used as a starting point for calculations. Taking these reaction steps into consideration during the calculations leads to significantly more reliable theoretical results, which can be applied either for prediction of the catalytic activity of yet untested species or for justification of the catalytic paths based upon the experimental kinetic data as well.
Based upon the experimentally obtained kinetic data on iodonium salt catalyzed nucleophilic addition of isocyanide to imine leading to imidazopyridine species, a reliable model for DFT calculations has been suggested. It was shown that preassociation of the catalysts with the reaction species might significantly affect the total energy profile of the reaction obtained by DFT. The associates of the organocatalysts featuring the solvent molecules ligated to its sigma-holes should be used as a starting point for calculations. Taking these reaction steps into consideration during the calculations leads to significantly more reliable theoretical results, which can be applied either for prediction of the catalytic activity of yet untested species or for justification of the catalytic paths based upon the experimental kinetic data as well.
Q1 Novikov A.S., Bolotin D.S., Halonium, Chalconium, and Pnictonium Salts as Noncovalent Organocatalysts: A Computational Study on Relative Catalytic Activity, Org. Biomol. Chem., 2022, 20, 7632–7639. DOI: 10.1039/D2OB01415G
 This theoretical study sheds light on the relative catalytic activity of pnictonium, chalconium, and halonium salts in reactions involving elimination of chloride and electrophilic activation of a carbonyl group. DFT calculations indicate that for cationic aromatic onium salts, values of the electrostatic potential on heteroatom σ-holes gradually increase from pnictogen- to halogen-containing species. The higher values of the potential on the halogen atoms of halonium salts result in the overall higher catalytic activity of these species, but in the case of pnictonium and chalconium cations, weak interactions from the side groups provide an additional stabilization effect on the reaction transition states. Based upon quantum-chemical calculations, the catalytic activity of phosphonium(V) and arsenonium(V) salts is expected to be too low to obtain effective noncovalent organocatalytic compounds, whereas stibonium(V), telluronium(IV) and iodonium(III) salts exhibit higher potential in application as noncovalent organocatalysts.
This theoretical study sheds light on the relative catalytic activity of pnictonium, chalconium, and halonium salts in reactions involving elimination of chloride and electrophilic activation of a carbonyl group. DFT calculations indicate that for cationic aromatic onium salts, values of the electrostatic potential on heteroatom σ-holes gradually increase from pnictogen- to halogen-containing species. The higher values of the potential on the halogen atoms of halonium salts result in the overall higher catalytic activity of these species, but in the case of pnictonium and chalconium cations, weak interactions from the side groups provide an additional stabilization effect on the reaction transition states. Based upon quantum-chemical calculations, the catalytic activity of phosphonium(V) and arsenonium(V) salts is expected to be too low to obtain effective noncovalent organocatalytic compounds, whereas stibonium(V), telluronium(IV) and iodonium(III) salts exhibit higher potential in application as noncovalent organocatalysts.
Q1 Il'in M.V., Novikov A.S., Bolotin D.S., Sulfonium and Selenonium Salts as Noncovalent Organocatalysts for the Multicomponent Groebke–Blackburn–Bienaymé Reaction, J. Org. Chem., 2022, 87, 10199–10207. DOI: 10.1021/acs.joc.2c01141
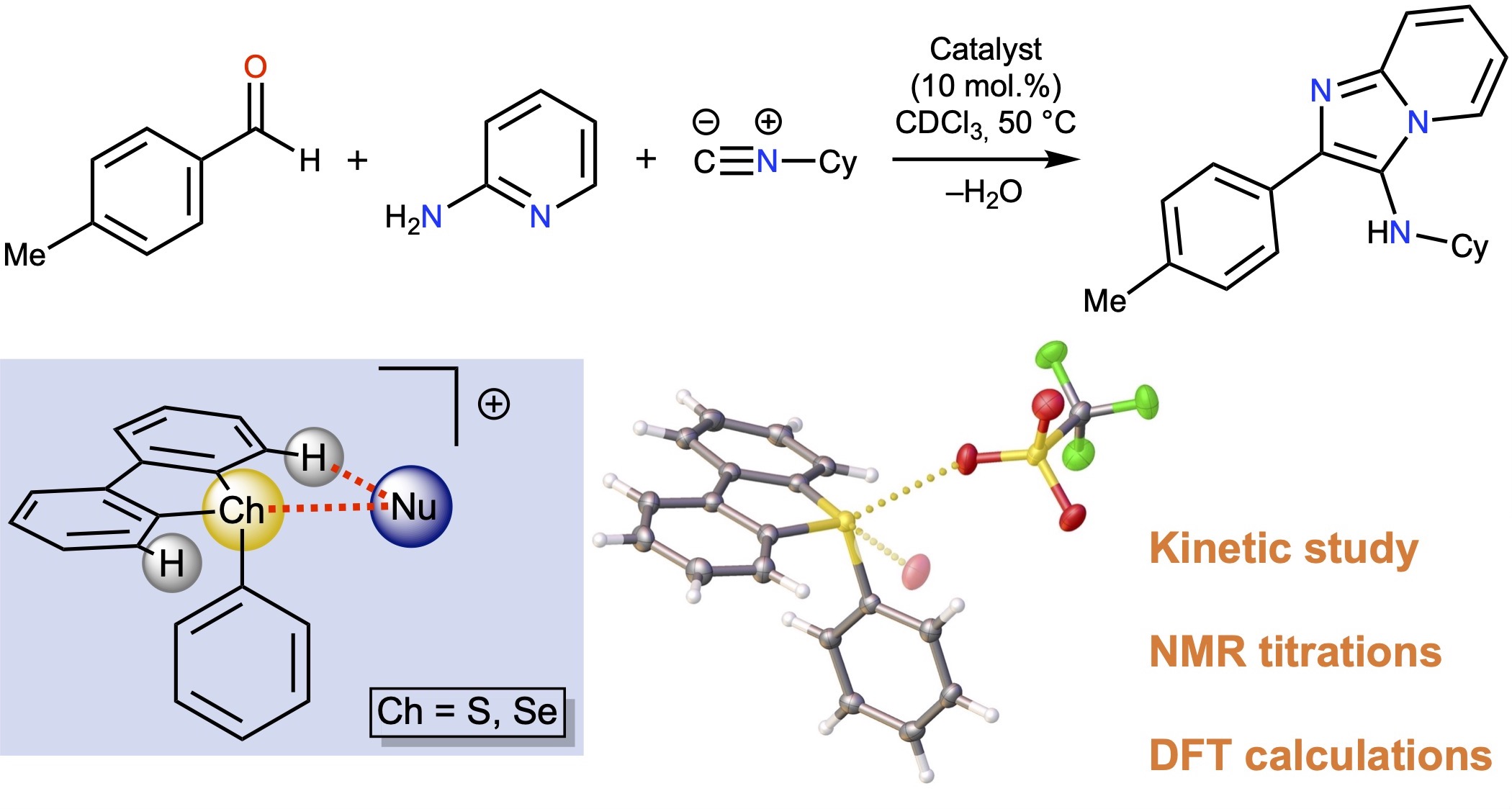 Sulfonium and selenonium salts, represented by S-aryl dibenzothiophenium and Se-aryl dibenzoselenophenium triflates, were found to exhibit remarkable catalytic activity in the model Groebke–Blackburn–Bienaymé reaction. Kinetic analysis and density functional theory (DFT) calculations indicated that their catalytic effect is induced by the ligation of the reaction substrates to the σ-holes on the S or Se atom of the cations. The experimental data indicated that although 10-fold excess of the chloride totally inhibits the catalytic activity of the sulfonium salts, the selenonium salt remains catalytically active, which can be explained by the experimentally found lower binding constant of the selenonium derivative to chloride in comparison with the sulfonium analogue. Both types of salts exhibit lower catalytic activity in the model reaction than dibenziodolium species.
Sulfonium and selenonium salts, represented by S-aryl dibenzothiophenium and Se-aryl dibenzoselenophenium triflates, were found to exhibit remarkable catalytic activity in the model Groebke–Blackburn–Bienaymé reaction. Kinetic analysis and density functional theory (DFT) calculations indicated that their catalytic effect is induced by the ligation of the reaction substrates to the σ-holes on the S or Se atom of the cations. The experimental data indicated that although 10-fold excess of the chloride totally inhibits the catalytic activity of the sulfonium salts, the selenonium salt remains catalytically active, which can be explained by the experimentally found lower binding constant of the selenonium derivative to chloride in comparison with the sulfonium analogue. Both types of salts exhibit lower catalytic activity in the model reaction than dibenziodolium species.
![]() Q1 Il'in M.V., Sysoeva A.A., Novikov A.S., Bolotin D.S., Diaryliodoniums as Hybrid Hydrogen- and Halogen-Bond-Donating Organocatalysts for the Groebke–Blackburn–Bienaymé Reaction. J. Org. Chem., 2022, 87, 4569–4579. DOI: 10.1021/acs.joc.1c02885
Q1 Il'in M.V., Sysoeva A.A., Novikov A.S., Bolotin D.S., Diaryliodoniums as Hybrid Hydrogen- and Halogen-Bond-Donating Organocatalysts for the Groebke–Blackburn–Bienaymé Reaction. J. Org. Chem., 2022, 87, 4569–4579. DOI: 10.1021/acs.joc.1c02885
 Dibenziodolium and diphenyliodonium triflates display high catalytic activity for the multicomponent reaction that leads to a series of imidazopyridines. Density functional theory (DFT) calculations indicate that both the salts can play the role of hybrid hydrogen- and halogen-bond-donating organocatalysts, which electrophilically activate the carbonyl and imine groups during the reaction process. The ortho-H atoms in the vicinal position to the I atom play a dual role: forming additional noncovalent bonds with the ligated substrate and increasing the maximum electrostatic potential on the σ-hole at the iodine atom owing to the effects of polarization. Dibenziodolium triflate exhibits higher catalytic activity, and the results obtained from 1H nuclear magnetic resonance (NMR) titrations, in conjunction with those from DFT calculations, indicate that this could be explained in terms of the additional energy required for the rotation of the phenyl ring in the diphenyliodonium cation during ligation of the substrate.
Dibenziodolium and diphenyliodonium triflates display high catalytic activity for the multicomponent reaction that leads to a series of imidazopyridines. Density functional theory (DFT) calculations indicate that both the salts can play the role of hybrid hydrogen- and halogen-bond-donating organocatalysts, which electrophilically activate the carbonyl and imine groups during the reaction process. The ortho-H atoms in the vicinal position to the I atom play a dual role: forming additional noncovalent bonds with the ligated substrate and increasing the maximum electrostatic potential on the σ-hole at the iodine atom owing to the effects of polarization. Dibenziodolium triflate exhibits higher catalytic activity, and the results obtained from 1H nuclear magnetic resonance (NMR) titrations, in conjunction with those from DFT calculations, indicate that this could be explained in terms of the additional energy required for the rotation of the phenyl ring in the diphenyliodonium cation during ligation of the substrate.
2021
![]() Q1 Sysoeva A.A., Novikov A.S., Il'in M.V., Suslonov V.V., Bolotin D.S., Predicting the catalytic activity of azolium-based halogen bond donors: an experimentally-verified theoretical study. Org. Biomol. Chem., 2021, 19, 7611–7620. DOI: 10.1039/D1OB01158H
Q1 Sysoeva A.A., Novikov A.S., Il'in M.V., Suslonov V.V., Bolotin D.S., Predicting the catalytic activity of azolium-based halogen bond donors: an experimentally-verified theoretical study. Org. Biomol. Chem., 2021, 19, 7611–7620. DOI: 10.1039/D1OB01158H
 This report demonstrates the successful application of electrostatic surface potential distribution analysis for evaluating the relative catalytic activity of a series of azolium-based halogen bond donors. A strong correlation (R2 > 0.97) was observed between the positive electrostatic potential of the σ-hole on the halogen atom and the Gibbs free energy of activation of the model reactions (i.e., halogen abstraction and carbonyl activation). The predictive ability of the applied approach was confirmed experimentally. It was also determined that the catalytic activity of azolium-based halogen bond donors was generally governed by the structure of the azolium cycle, whereas the substituents on the heterocycle had a limited impact on the activity. Ultimately, this study highlighted four of the most promising azolium halogen bond donors, which are expected to exhibit high catalytic activity.
This report demonstrates the successful application of electrostatic surface potential distribution analysis for evaluating the relative catalytic activity of a series of azolium-based halogen bond donors. A strong correlation (R2 > 0.97) was observed between the positive electrostatic potential of the σ-hole on the halogen atom and the Gibbs free energy of activation of the model reactions (i.e., halogen abstraction and carbonyl activation). The predictive ability of the applied approach was confirmed experimentally. It was also determined that the catalytic activity of azolium-based halogen bond donors was generally governed by the structure of the azolium cycle, whereas the substituents on the heterocycle had a limited impact on the activity. Ultimately, this study highlighted four of the most promising azolium halogen bond donors, which are expected to exhibit high catalytic activity.
![]() Q1 Yunusova S.N., Novikov A.S., Soldatova N.S., Vovk M.A., Bolotin D.S. Iodonium salts as efficient iodine(III)-based noncovalent organocatalysts for Knorr-type reactions. RSC Adv., 2021, 11, 4574–4583. DOI: 10.1039/d0ra09640g
Q1 Yunusova S.N., Novikov A.S., Soldatova N.S., Vovk M.A., Bolotin D.S. Iodonium salts as efficient iodine(III)-based noncovalent organocatalysts for Knorr-type reactions. RSC Adv., 2021, 11, 4574–4583. DOI: 10.1039/d0ra09640g
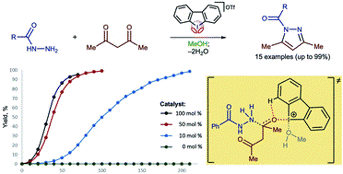 Hypervalent iodine(III)-derivatives display higher catalytic activity than other aliphatic and aromatic iodine(I)– or bromine(I)-containing substrates for a Knorr-type reaction of N-acetyl hydrazides with acetyl acetone to give N-acyl pyrazoles. The highest activity was observed for dibenziodolium triflate, for which 10 mol% resulted in the generation of N-acyl pyrazole from acyl hydrazide and acetyl acetone typically at 50 C for 3.5–6 h with up to 99% isolated yields. 1H NMR titration data and DFT calculations indicate that the catalytic activity of the iodine(III) is caused by the binding with a ketone.
Hypervalent iodine(III)-derivatives display higher catalytic activity than other aliphatic and aromatic iodine(I)– or bromine(I)-containing substrates for a Knorr-type reaction of N-acetyl hydrazides with acetyl acetone to give N-acyl pyrazoles. The highest activity was observed for dibenziodolium triflate, for which 10 mol% resulted in the generation of N-acyl pyrazole from acyl hydrazide and acetyl acetone typically at 50 C for 3.5–6 h with up to 99% isolated yields. 1H NMR titration data and DFT calculations indicate that the catalytic activity of the iodine(III) is caused by the binding with a ketone.
Department of Organic Chemistry
Research Group of Professor Irina Balova
Short URL for media go.spbu.ru/rgbalova
- Acetylenic and diacetylenic compounds in organic synthesis
- Synthesis of heterocycles and ethynylheterocycles
- Synthesis of acyclic and cyclic enediynes
- New catalysts for the synthesis of acetylenic compounds
- Flow chemistry and microreactor synthesis
- Development of new protein tyrosine phosphatase 1B inhibitors based on 4-oxo-1,4-dihydrocinnoline derivatives

Group Members
 |
Group Leader Balova Irina A.Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Danilkina Natalia A.Ph.D., Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Efremova MariyaPh.D., Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Govdi Anastasia I.Ph.D., Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Sorokoumov Viktor N.Ph.D., Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Kaminsky NikitaMSc, Research Engineer This email address is being protected from spambots. You need JavaScript enabled to view it.
|
Students
 |
Khmelevskaya Ekaterinapostgraduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Vidyakina Aleksandrapostgraduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Didenko Egor2 year graduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Kim Mia1 year graduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Ogurtsova Anna1 year graduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Korolyov-Zelyoniy Kirill4 year undergraduate student |
 |
Kutuzov Yaroslav4 year undergraduate student |
 |
Galkin Egor3 year undergraduate student |
 |
Evsyukov Ilya2 year undergraduate student |
 |
Hashimova Diana2 year undergraduate student |
 |
Menchikov Vasiliy2 year undergraduate student |
 |
Melnikov Vladimir1 year undergraduate student |
Research Topic 1
Acetylene compounds in organic synthesis and for the construction of compounds with useful properties
(A) Synthetic approaches toward functionalized diacetylenes
Among different known synthetic routes toward terminal diynes «diacetylene zipper» reaction, which has been discovered in our research group, is of special importance. [A] It is a unique process of simultaneous migration of two conjugated triple bonds from an internal to a terminal position promoted by lithium 2-aminoethanamide. The scope of the reaction is unfunctionalized alkadiynes along with diacetylenic alcohols. [B][1]
«Diacetylene zipper» reaction followed by functionalization of lithium acetylides was used in the synthesis of diacetylenic alcohols [C][D], ketones, [2] carboxylic acids, [D] tetraynes [E] and other derivatives.
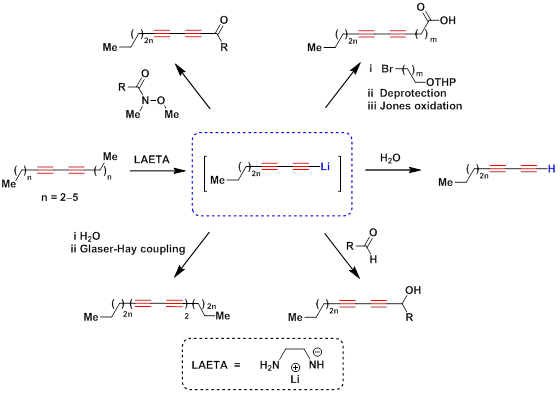
Being combined with the Sonogashira coupling, «diacetylene zipper» reaction was used as a general-purpose method for the synthesis of aryl- and heteroaryldiacetylenes. [3][4][5][6][1][7][8]
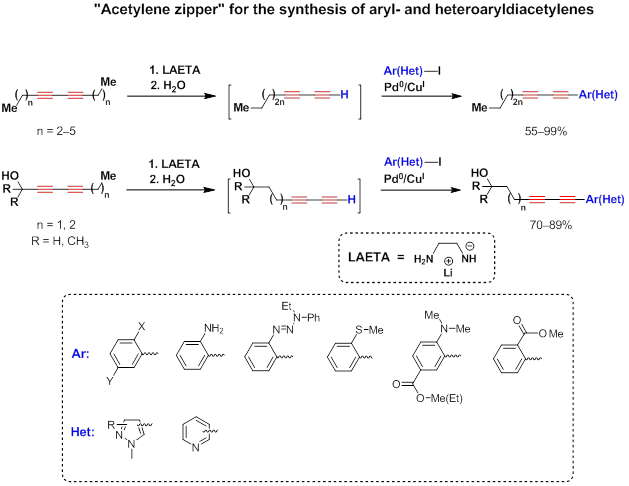
Removing of protecting groups is known to be the second route toward terminal diacetylenic compounds. Trimethylsilyl (TMS) group can be deprotected under mild conditions. Therefore desilylation of TMS-diacetylenes using potassium fluoride (KF) and the Sonogashira coupling being held in one-pot was found to be very convenient for the synthesis of functionalized arenes bearing buta-1,3-diynyl moiety. [9]
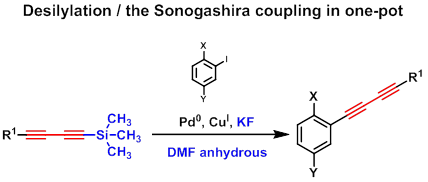
(B) Diacetylenic compounds in the synthesis of heterocycles
Two conjugated triple bonds are endowed with a unique synthetic potential. The research in our group revealed that aryldiacetylenes with a heteroatom functional group at ortho-position with two conjugated triple bonds are able to undergo heterocyclization (electrophile-induced cyclization, [10][11][12] and the Richter-type cyclization [5][13][14]) through only one of two triple bonds, remaining the second one untouched. These transformations afford heterocycles with a triple bond and a halogen atom at neighboring С atoms.
The method described was used for the synthesis of iodoethynylbenzothiophenes, -benzofurans, -indoles, -isocoumarines and 4-bromo(chloro)cinnolines.
Recently we have also discovered that Cu-catalyzed azide-alkyne cycloaddition (CuAAC) of 1-iodobuta-1,3-diynes with organic azides is a high-yielded and functional group tolerant synthetic method for the preparation of 5-iodo-4-ethynyl-1H-[1,2,3]triazoles. [15]
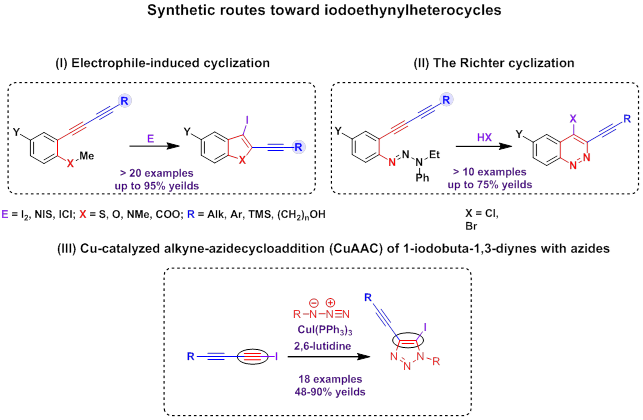
Both functional groups (a triple bond and a halogen atom) are of special importance for further functionalization of a heterocycle formed. Based on synthetic techniques for the synthesis of iodoethynylheterocycles we have elaborated a three-step synthetic route toward heterocycle-fused analogues of naturally-occurring enediyne antibiotics.
(C) Synthesis and properties of enediyne antibiotics analogues
Enediyne antibiotics are compounds with a high level of anticancer and antibacterial activity caused by a special structural feature – (Z)-hexa-3-en-1,5-diyne system. Being incorporated into a 9- or 10-membered cycle, an enediyne moiety is able to undergo the Bergman cyclization at ambient temperatures with the formation of highly reactive 1,4-phenylene diradicals which can damage DNA.
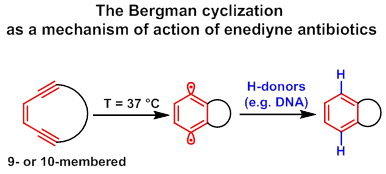
We focus our research on the search of simple synthetic analogues of enediyne antibiotics among enediyne systems fused to a heterocycle. The synthetic approach toward iodoethynylheterocycles in cooperation with the Sonogashira coupling allowed acetylene moieties with specially functional groups to be introduced into a heterocycle formed at neighboring C atoms. In turn, it provides an opportunity for the synthesis of special starting acyclic enediyne compounds for the next macrocyclization step. Different synthetic macrocyclization tools for the synthesis of cyclic enediynes have been tested in our group: the Nozaki-Hiyama-Kishi reaction, [16][17] ring-closing metathesis (RCM) [11][18] and the Nicholas reaction. [19][20][21]
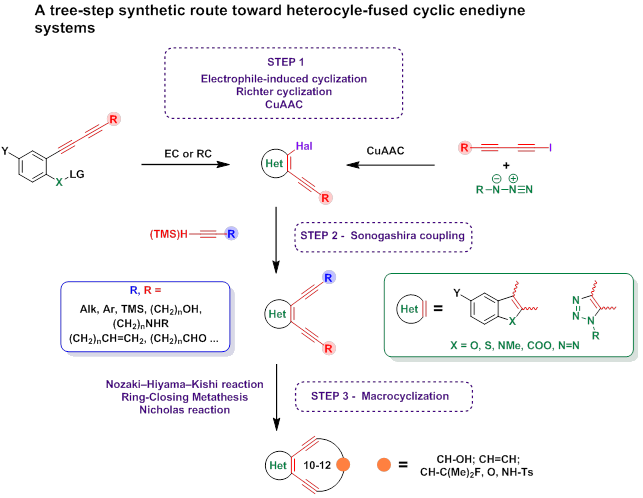
The Nicholas reaction as a macrocyclization tool was found to be the most promising and universal technique for these purposes. Thus several 10-membered enediynes fused to a benzothiophene have been synthesized and used for the creation and verification of the scale of relative reactivity of 10-membered enediynes. [20]
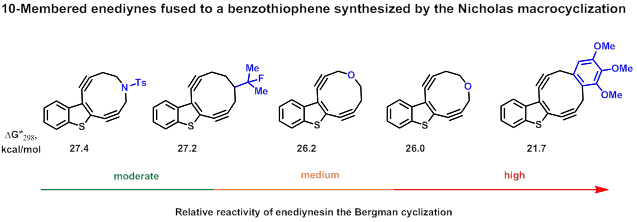
In collaboration with the Department of Genetics and Biotechnology of SPbU we have estimated that 10-membered benzothiophene-fused enediynes are able to cleave plasmid DNA inducing both single and double strand breaks. This property of enediynes is of great importance for the search of new anticancer compounds within benzothiophene-fused enediyne family.
Today we are working at the synthesis of small library of 10-membered enediynes fused to other heterocycles.
(D) Synthesis of new fluorescent poly(arylene ethynylene)s
Nowadays poly(arylene ethynylene)s represents a promising type of compounds for the construction of polymers with precise desired properties. Thus for poly(arylene ethynylene)s bearing special functional groups responsible for an analyte binding, a fluorescence quenching effect can be detected as a response for the interaction with the analyte molecule. Such polymers are of great interest for the creation of new detecting systems. We have synthesized poly(arylene ethynylene)s with cinnoline rings in a polymer chain. The fluorescence quenching effect was detected as a special response for PdII ions.[22]
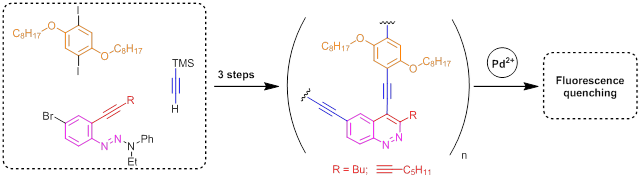
References
- L. A. Remizova, I. A. Balova, and I. A. Favorskaya, Prototropic isomerization of diacetylenic compounds. Zh. Org. Khim., 1986, 22, 2459 (in Russian).
- I. A. Balova, I. V. Zakharova, and L. A. Remizova. Studies on the Prototropic Isomerization of Diacetylene Alcohols. Russ. J. Org. Chem. (Engl. Transl.) 1993, 29, 1439.
- Balova, I. A.; Morozkina, S. N.; Voskresenskii, S. V.; Remizova, L. A. Consecutive reactions of diacetylenes: "Acetylene zipper" reaction and hydroxyalkylation of lithium 1,3-alkadiynides as a synthetic route to α- and β-long-chain diacetylenic alcohols. Russ. J. Org. Chem. (Engl. Transl.) 2000, 36 (10), 1428.
- Balova, I. A.; Remizova, L. A.; Makarycheva, V. F.; Rumyantseva, E. G.; Favorskaya, I. A. Russ. J. Org. Chem. (Engl. Transl.) 1991, 27, 55.
- Balova, I. A., Remizova, L.A. «The "Acetylene Zipper" Reaction of Diacetylene Compounds in the Synthesis of Tetraynes» Russ. J. Org. Chem. (Engl. Transl.) 1994, 30, (2) 213.
- Kulyashova, A.E.; Sorokoumov, V.N.; Popik, V. V; Balova, I.A. An acetylene zipper—Sonogashira reaction sequence for the efficient synthesis of conjugated arylalkadiynols. Tetrahedron Lett. 2013, 54, 2235–2238, https://linkinghub.elsevier.com/retrieve/pii/S004040391300316X
- Novikov, R. V; Vasil’ev, A.A.; Balova, I.A. Convenient synthesis of dodeca-1,3-diynyl ketones by the “diacetylene zipper” reaction. Russ. Chem. Bull. 2005, 54, 1043–1045, http://link.springer.com/10.1007/s11172-005-0355-8
- Balova, I.A.; Morozkina, S.N.; Knight, D.W.; Vasilevsky, S.F. A one-pot synthesis of 1-arylalka-1,3-diynes by sequential acetylene zipper and Sonogashira reactions. Tetrahedron Lett. 2003, 44, 107–109, http://linkinghub.elsevier.com/retrieve/pii/S0040403902024966
- Balova, I.A.; Sorokoumov, V.N.; Morozkina, S.N.; Vinogradova, O. V; Knight, D.W.; Vasilevsky, S.F. A Convenient Synthesis of Functionalised 1-Aryl-1,3-alkadiynes. Eur. J. Org. Chem. 2005, 2005, 882–888, http://doi.wiley.com/10.1002/ejoc.200400688
- Vinogradova, O. V.; Sorokoumov, V.N.; Balova, I.A. A short route to 3-alkynyl-4-bromo(chloro)cinnolines by Richter-type cyclization of ortho-(dodeca-1,3-diynyl)aryltriaz-1-enes. Tetrahedron Lett. 2009, 50, 6358–6360, http://dx.doi.org/10.1016/j.tetlet.2009.08.103
- Sorokoumov, V.N.; Popik, V. V.; Balova, I.A. Access to 2,3-bis(buta-1,3-diynyl)pyridines. Mendeleev Commun. 2011, 21, 19–20, https://linkinghub.elsevier.com/retrieve/pii/S0959943611000095
- Govdi, A.I.; Kulyashova, A.E.; Vasilevsky, S.F.; Balova, I.A. Functionalized buta-1,3-diynyl- N -methylpyrazoles by sequential “diacetylene zipper” and Sonogashira coupling reactions. Tetrahedron Lett. 2017, 58, 762–765, http://dx.doi.org/10.1016/j.tetlet.2017.01.032
- Kulyashova, A.E.; Mikheeva, E. V.; Danilkina, N.A.; Balova, I.A. Synthesis of 2-(buta-1,3-diynyl)-N,N-dimethylanilines Using Reductive Methylation Step. Mendeleev Commun. 2014, 24, 102–104, http://dx.doi.org/10.1016/j.mencom.2014.03.013
- Lyapunova, A.G.; D’yachenko, A.S.; Danilkina, N.A. Potassium fluoride for one-pot desilylation and the Sonogashira coupling of ethynylsilanes and buta-1,3-diynylsilanes. Russ. J. Org. Chem. 2017, 53, 800–804, http://link.springer.com/10.1134/S1070428017050268
- Danilkina, N.; Bräse, S.; Balova, I. Electrophilic Cyclization of Buta-1,3-diynylarenes: Synthesis of Precursors of (Z)-3-Ene-1,5-diyne Systems Fused to Heterocycles. Synlett 2011, 2011, 517–520, http://www.thieme-connect.de/DOI/DOI?10.1055/s-0030-1259547
- Danilkina, N.A.; Kulyashova, A.E.; Khlebnikov, A.F.; Bräse, S.; Balova, I.A. Electrophilic Cyclization of Aryldiacetylenes in the Synthesis of Functionalized Enediynes Fused to a Heterocyclic Core. J. Org. Chem. 2014, 79, 9018–9045, http://pubs.acs.org/doi/10.1021/jo501396s
- Danilkina, N.A.; Gurskaya, L.Y.; Vasilyev, A. V.; Balova, I.A. Towards Isocoumarin-Fused Enediyne Systems through the Electrophilic Cyclization of Methyl o -(Buta-1,3-diynyl)benzoates. Eur. J. Org. Chem. 2016, 2016, 739–747, http://doi.wiley.com/10.1002/ejoc.201501262
- Vinogradova, O. V.; Sorokoumov, V.N.; Vasilevsky, S.F.; Balova, I.A. The Richter reaction of ortho-(alka-1,3-diynyl)aryldiazonium salts. Tetrahedron Lett. 2007, 48, 4907–4909, https://linkinghub.elsevier.com/retrieve/pii/S0040403907009203
- Vinogradova, O. V.; Sorokoumov, V.N.; Vasilevskii, S.F.; Balova, I.A. Studies on cyclization of o-(alka-1,3-diynyl)arenediazonium salts. Russ. Chem. Bull. 2008, 57, 1725–1733, http://link.springer.com/article/10.1007/s11172-008-0228-z
- Govdi, A.I.; Danilkina, N.A.; Ponomarev, A. V.; Balova, I.A. 1-Iodobuta-1,3-diynes in Copper-Catalyzed Azide–Alkyne Cycloaddition: A One-Step Route to 4-Ethynyl-5-iodo-1,2,3-triazoles. J. Org. Chem. 2019, 84, 1925–1940, http://pubs.acs.org/doi/10.1021/acs.joc.8b02916
- Kulyashova, A.E.; Ponomarev, A.V.; Selivanov, S.I.; Khlebnikov, A.F.; Popik, V.V.; Balova, I.A. Cr(II)-promoted internal cyclization of acyclic enediynes fused to benzo[ b ]thiophene core: Macrocycles versus 2-methylenecycloalkan-1-ols formation. Arab. J. Chem. 2018, https://doi.org/10.1016/j.arabjc.2018.05.005
- Vinogradova, O. V.; Balova, I.A.; Popik, V. V. Synthesis and Reactivity of Cinnoline-Fused Cyclic Enediyne. J. Org. Chem. 2011, 76, 6937–6941, http://pubs.acs.org/doi/abs/10.1021/jo201148h
- Danilkina, N.; Nieger, M.; Selivanov, S.; Bräse, S.; Balova, I. Electrophilic Cyclization and Ring-Closing Metathesis as Key Steps in the Synthesis of a 12-Membered Cyclic Enediyne. Eur. J. Org. Chem. 2012, 2012, 5660–5664, http://doi.wiley.com/10.1002/ejoc.201200881
- Lyapunova, A.G.; Danilkina, N.A.; Khlebnikov, A.F.; Köberle, B.; Bräse, S.; Balova, I.A. Oxaenediynes through the Nicholas-Type Macrocyclization Approach. Eur. J. Org. Chem. 2016, 2016, 4842–4851, http://doi.wiley.com/10.1002/ejoc.201600767
- Lyapunova, A.G.; Danilkina, N.A.; Rumyantsev, A.M.; Khlebnikov, A.F.; Chislov, M. V.; Starova, G.L.; Sambuk, E. V.; Govdi, A.I.; Bräse, S.; Balova, I.A. Relative Reactivity of Benzothiophene-Fused Enediynes in the Bergman Cyclization. J. Org. Chem. 2018, 83, 2788–2801, http://pubs.acs.org/doi/10.1021/acs.joc.7b03258
- Danilkina, N.; Rumyantsev, A.; Lyapunova, A.; D’yachenko, A.; Khlebnikov, A.; Balova, I. 10-Membered Azaenediyne Fused to a Benzothiophene through the Nicholas Macrocyclization: Synthesis and DNA Cleavage Ability. Synlett 2019, 30, 161–166, http://www.thieme-connect.de/DOI/DOI?10.1055/s-0037-1610352
- Danilkina, N.A.; Vlasov, P.S.; Vodianik, S.M.; Kruchinin, A.A.; Vlasov, Y.G.; Balova, I.A. Synthesis and chemosensing properties of cinnoline-containing poly(arylene ethynylene)s. Beilstein J. Org. Chem. 2015, 11, 373–384, http://www.beilstein-journals.org/bjoc/content/11/1/43
Research Topic 2
The new catalysts and their application in the synthesis of acetylenic compounds
For the effective implementation of "atom-economy" transformations in the creation of new carbon-carbon and carbon-heteroatom bonds within acetylene derivatives and other organic compounds, our group conduct research aimed on the development of effective homogeneous and heterogeneous catalytic systems.
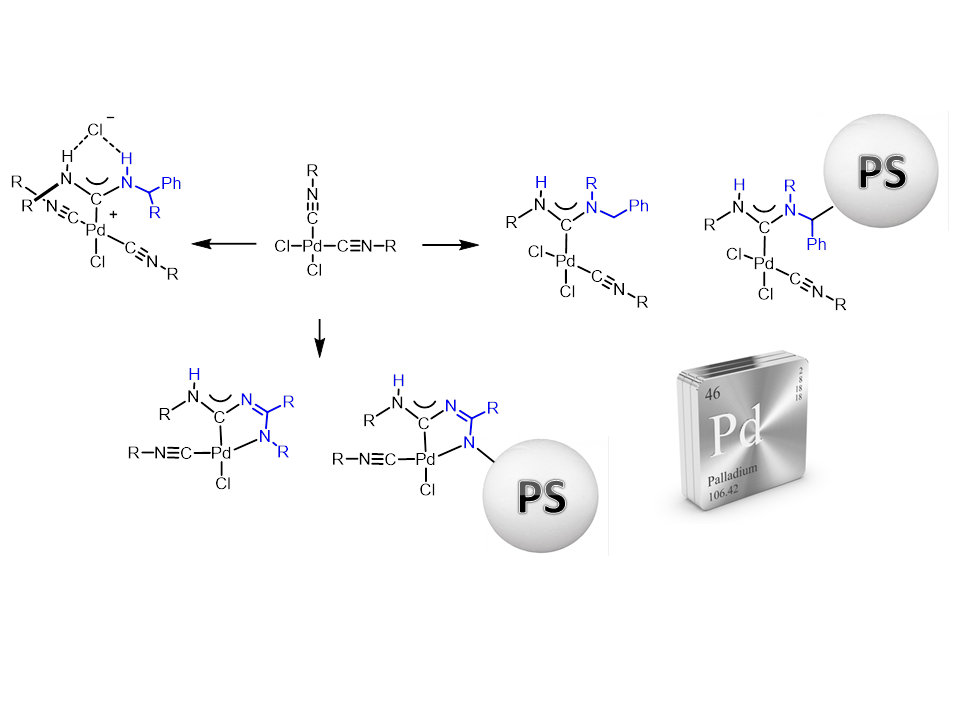
We can separated our work into two interdependent directions:
- The investigation of highly-active homogeneous catalysts based on palladium diaminocarbene complexes which are capable to work effectively under low catalytic concentrations conditions;
- The development of the reusable catalysts by immobilization of highly active homogeneous palladium complexes on a suitable support, which is inert under the reaction conditions used.
Currently, a method for the efficient heterogeneous catalytic systems preparation based on acyclic palladium diaminocarbene complexes has been developed using the metal-promoted reaction of nucleophilic addition of amino- and amidine- groups to isocyanides in palladium(II) complexes. The catalyst has high activity for the Sonogashira and Suzuki reactions on a wide set of substrates with the possibility of multiple use without significant loss of catalytic activity.
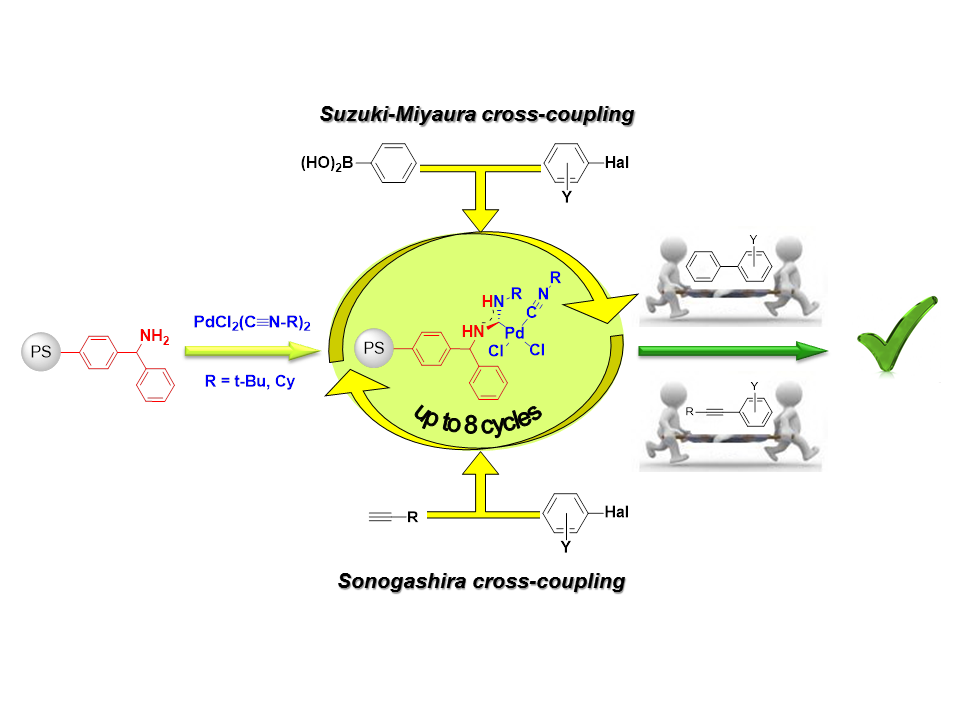
We have shown that the main contribution to the catalytic activity of heterogeneous precatalysts based on palladium acyclic diaminocarbene complexes is made by the palladium reversibly leached into the solution from the support during the reaction due to a new pathway of Pd(II) – Pd(0) activation through the carbodiimide formation. Our findings show the principally new route for Pd0-species generation and stabilization, and casts light on the nature of the catalysis for Pd(II)-complexes with different diaminocarbene ligand.
Finally, the ease of carbodiimide and HCl elimination from the certain ADCs-Pd with the formation of Pd(0) can be utilized for the catalyst activation in the manner used for commercially available Buchwald precatalysts.
Our research team is also developing advanced catalytic systems which can catalyze low-reactive commercially important aryl chlorides under mild conditions (at room temperature without using strong bases).
Research Topic 3
The development of the new inhibitors of protein phosphotyrosine phosphatase 1B (PTP1B)
The enzyme protein phosphotyrosine phosphatase 1B (PTP1B) is the most important negative regulator of the insulin and leptin systems functioning through the activation of a cascade of tyrosine phosphorylation. The increased activity of PTP1B leads to the insulin (IR) and leptin resistances (LR) and is the cause of obesity, diabetes mellitus and other socially significant metabolic disorders. In this regard, an actual problem of modern medicine are deciphering the mechanisms of action of PTP1B and developing its selective inhibitors, which can allow treating the metabolic disorders associated with IR and LR. The most promising is the creation of "binary" PTP1B inhibitors that simultaneously interact with the catalytic (low-specific) and allosteric (high-specific) sites of PTP1B, which ensures the high efficiency and selectivity of their action.
We proposed to use derivatives of 4-oxo-1,4-dihydrocinnoline as specific inhibitors of PTP1B. The developed approach to the synthesis of 4-oxo-1,4-dihydroindolone using Sonogashira reaction to obtain ortho-ethynylsubstituted anilines and the Richter reaction for the formation of cinnoline core, allows obtaining of 4-oxo-1,4-dihydrocinnoline derivatives in good yields with a small number of steps. Were also obtained promising results about the effectiveness of the synthesized derivative of 4-oxo-1,4-dihydrocinnoline – ethyl ester 3-(hydroximethyl)-4-oxo-1,4-dihydrcinnoline-6-carboxylic acid as a specific inhibitor PTP1B in vitro and in vivo. It was shown that the treatment of the primary culture of rat hypothalamic neurons with ethyl 3-(hydroxymethyl)-4-oxo-1,4-dihydrocinnoline-6-carboxylate increases the stimulating effect of leptin on phosphorylation of Akt-kinase and STAT3-protein, which indicates the suppression of the activity of PTP1B, which negatively regulates the effects of leptin. Along with this, this derivative of 4-oxo-1,4-dihydrocinoline when administered to rats caused a decrease in appetite, body weight, adipose tissue, improved metabolic parameters and increased insulin sensitivity in rats with an experimental model of obesity.
Publications
2023
- A.A. Vidyakina, A. A. Shtyrov, M.N. Ryazantsev, A. F. Khlebnikov, I. E. Kolesnikov, V. V. Sharoyko. D. V. Spiridonova, I. A. Balova, S. Bräse, N. A. Danilkina. Development of Fluorescent Isocoumarin-Fused Oxacyclononyne – 1,2,3-Triazole Pairs. Chemistry — A European Journal, 2023, e202300540. Статья находится в открытом доступе и попала в Hot Topic: Click Chemistry издательства Wiley: 10.1002/(ISSN)1521-3773.hottopic-clickchemistry.
- A. A. Babushkina, V. N. Mikhailov. A. D. Ogurtsova, A. S. Bunev, V.N. Sorokoumov, I. A. Balova. The Richter reaction in the synthesis of combretastatin analogs. Russian Chemical Bulletin. 2023, 72(4), 1012-1022. DOI: 10.1007/s11172-023-3866-3.
- A. I. Govdi, S. O. Anisimov, N. A. Danilkina, A. S. Bunev. I. A. Balova. Acyclic enediynes fused to triazoleand benzothiophene containing propargylamine moieties. Mendeleev Commun., 2023, 33, 328–330. DOI: 10.1016/j.mencom.2023.04.010.
- K. V. Derkach, M. A. Gureev, A. A. Babushkina, V. N. Mikhaylov , I. O. Zakharova, A. A. Bakhtyukov, V. N. Sorokoumov, A. S. Novikov, M. Krasavin, A. O. Shpakov, I. A. Balova. Dual PTP1B/TC-PTP Inhibitors: Biological Evaluation of 3-(Hydroxymethyl)cinnoline-4(1H)-Ones, Int. J. Mol. Sci. 2023, 24(5), 4498; DOI: 10.3390/ijms24054498.
- Bakhtyukov A.A., Derkach K.V., Fokina E.A. , Lebedev I.A., Sorokoumov V.N., Bayunova, L.V., Shpakov A.O., / Effect of Different Luteinizing Hormone Receptor Agonists on Ovarian Steroidogenesis in Mature Female Rats. / J Evol Biochem Phys 59, 57–68 (2023). DOI: 10.1134/S0022093023010052.
- Fokina E.A., Derkach K.V., Bakhtuykov A.A., Sorokoumov V.N., Lebedev I.A., Morina I.Y., Shpakov A.O. / Stimulation Of Ovulation In Immature Female Rats Using Orthosteric And Allosteric Luteinizing Hormone Receptor Agonists / Doklady Rossijskoj akademii nauk. Nauki o žizni. – (2023). - Vol. 508. - N. 1. - P. 30-34. doi: 10.31857/S2686738922700032.
- Efremova M.М., Rumyantsev A.M., Babitova E.S., Ianshina T. M., Govd A.I. Synthesis of 5-ethynylisoxazoles based on 1,3-dipolar cycloaddition reactions of nitrile oxides with conjugated diynes. Russ. Chem. Bull. 2023, 72, 1717–1721. DOI: 10.1007/s11172-023-3952-5.
- T. Ianshina; A. Sidorin; K. Petrova; M. Shubert, A. Makeeva, E. Sambuk; A. Govdi, A. Rumyantsev, M. Padkina Effect of Methionineon Gene Expression in Komagataella phaffii Cells. Microorganisms. 2023, 11, 877. DOI: 10.3390/microorganisms11040877.
- S.G.Zlotin, K.S.Egorova, V.P.Ananikov, A.A.Akulov, M.V.Varaksin, O.N.Chupakhin, V.N.Charushin...., I.A.Balova, V.N.Sorokoumov et al. Russ. Chem. Rev., 2023, 92 (12) RCR5104.
- Derkach, K.V.; Lebedev, I.A.; Morina, I.Y.; Bakhtyukov, A.A.; Pechalnova, A.S.; Sorokoumov, V.N.; Kuznetsova, V.S.; Romanova, I.V.; Shpakov, A.O., Int. J. Mol. Sci., 24 (2023), 16618.
2022
- Danilkina, N.A.; Khmelevskaya, E.A.; Lyapunova, A.G.; D’yachenko, A.S.; Bunev, A.S.; Gasanov, R.E.; Gureev, M.A.; Balova, I.A. Functionalized 10-Membered Aza- and Oxaenediynes through the Nicholas Reaction. Molecules 2022, 27, 6071, doi: 10.3390/molecules27186071. Impact Factor: 4.927.
- Babushkina, A.A.; Mikhaylov, V.N.; Novikov, A.S.; Sorokoumov, V.N.; Gureev, M.A.; Kryukova, M.A.; Shpakov, A.O.; Balova, I.A. Synthesis , X-ray and DFT studies of 6-halo-3-(hydroxymethyl ) cinnolin-4(1H)-ones. Chem. Heterocycl. Compd. 2022, 58, 432–437, doi: 10.1007/s10593-022-03109-3. Impact Factor: 1.490.
- Govdi, A.I.; Tokareva, P.V.; Rumyantsev, A.M.; Panov, M.S.; Stellmacher, J.; Alexiev, U.; Danilkina, N.A.; Balova, I.A. 4,5-Bis(arylethynyl)-1,2,3-triazoles—A New Class of Fluorescent Labels: Synthesis and Applications. Molecules 2022, 27, doi: 10.3390/molecules27103191. Impact Factor: 4.927.
- Gholinejad, M.; Shojafar, M.; Sansano, J.M.; Mikhaylov, V.N.; Balova, I.A.; Khezri, R. Hyperbranched polymer immobilized palladium nanoparticles as an efficient and reusable catalyst for cyanation of aryl halides and reduction of nitroarenes. J. Organomet. Chem. 2022, 970–971, doi: 10.1016/j.jorganchem.2022.122359. Impact Factor: 2.345.
- Derkach, K. V; Fokina, E.A.; Bakhtyukov, A.A.; Sorokoumov, V.N.; Stepochkina, A.M.; Zakharova, I.O.; Shpakov, A.O. The Study of Biological Activity of a New Thieno[2,3-D]-Pyrimidine-Based Neutral Antagonist of Thyrotropin Receptor. Bull. Exp. Biol. Med. 2022, 172, 713–717, doi: 10.1007/s10517-022-05462-x. Impact Factor: 0.737.
- Bakhtyukov, A.A.; Derkach, K. V; Fokina, E.A.; Sorokoumov, V.N.; Zakharova, I.O.; Bayunova, L. V; Shpakov, A.O. Development of Low-Molecular-Weight Allosteric Agonist of Thyroid-Stimulating Hormone Receptor with Thyroidogenic Activity. Dokl. Biochem. Biophys. 2022, 503, 67–70, doi: 10.1134/S1607672922020016. Impact Factor: 0.834.
- Bakhtyukov, A.A.; Derkach, K. V; Sorokoumov, V.N.; Stepochkina, A.M.; Romanova, I. V; Morina, I.Y.; Zakharova, I.O.; Bayunova, L. V; Shpakov, A.O. The effects of separate and combined treatment of male rats with type 2 diabetes with metformin and orthosteric and allosteric agonists of luteinizing hormone receptor on steroidogenesis and spermatogenesis. Int. J. Mol. Sci. 2022, 23, doi: 10.3390/ijms23010198. Impact Factor: 6.208.
- Bakhtyukov, A.A.; Derkach, K.V.; Stepochkina, A.M.; Sorokoumov, V.N.; Bayunova, L.V.; Lebedev, I.A.; Shpakov, A.O. Effects of metformin and lutheinizing hormone receptor agonists on steroidogenesis and spermatogenesis in rats with type 2 diabetes with their separate and combined administration. Metabolism 2022, 128, 155010, doi: 10.1016/j.metabol.2021.155010. Impact Factor: 13.934.
- Bakhtyukov, A.A.; Morina, I.Y.; Derkach, K. V.; Romanova, I. V.; Sorokoumov, V.N.; Shpakov, A.O. Development of Approaches to Reducing the Effective Gonadotropin Dose in Treating Androgen Insufficiency in Male Rats with Type 1 Diabetes Mellitus. J. Evol. Biochem. Physiol. 2022, 58, 1503–1513, doi: 10.1134/S0022093022050209. Impact Factor: 1.621.
- Stepochkina, A.M.; Bakhtyukov, A.A.; Derkach, K. V.; Sorokoumov, V.N.; Shpakov, A.O. A Comparative Study of the Steroidogenic Effect of 5-Amino-N-tert-butyl-2-(methylthio)-4-(3-(nicotinamido)phenyl)thieno[2,3-d]-pyrimidine-6-carboxamide and Chorionic Gonadotropin with Different Methods of Administration to Male Rats. J. Evol. Biochem. Physiol. 2022, 58, 54–63, doi: 10.1134/S0022093022010057. Impact Factor: 1.621.
2021
- Mikhaylov, V.N.; Kazakov, I. V.; Parfeniuk, T.N.; Khoroshilova, O. V.; Scheer, M.; Timoshkin, A.Y.; Balova, I.A. The carbene transfer to strong Lewis acids: copper is better than silver. Dalt. Trans. 2021, 50, 2872–2879. doi: 10.1039/D1DT00235J.
- Efremova, M.M.; Govdi, A.I.; Frolova, V.V.; Rumyantsev, A.M.; Balova, I.A. Design and Synthesis of New 5-aryl-4-Arylethynyl-1H-1,2,3-triazoles with Valuable Photophysical and Biological Properties. Molecules. 2021, 26, 2801. doi: 10.3390/molecules26092801.
- V. N. Mikhaylov, I. A. Balova. Alternative Transformations of N-Heterocyclic Carbene Complexes of the Group 11 Metals in Transmetalation Reactions (A Review). Russian Journal of General Chemistry. 2021, Vol. 91, No. 11, pp. 2192–2246. DOI: 10.1134/S1070363221110098.
- Danilkina N.A., Govdi A.I., Khlebnikov A. F., Tikhomirov A.O. Sharoyko V. V., Shtyrov A. A., Ryazantsev M. N. Bräse S., Balova I. A. Heterocycloalkynes Fused to a Heterocyclic Core: Searching for an Island with Optimal Stability-Reactivity Balance. J. Am. Chem. Soc. 2021, 143, 16519–16537. doi: 10.1021/jacs.1c06041.
- Danilkina, N.A.; Andrievskaya, E.V.; Vasileva, A.V.; Lyapunova, A.G.; Rumyantsev, A.M.; Kuzmin, A.A.; Bessonova, E.A.; Balova, I.A. 4-Azidocinnoline—Cinnoline-4-amine Pair as a New Fluorogenic and Fluorochromic Environment-Sensitive Probe. Molecules. 2021, 26, 7460. doi: 10.3390/molecules26247460.
- A.A. Bakhtyukov, K.V. Derkach, I.V. Romanova, V.N. Sorokoumov, T.V. Sokolova, A.I. Govdi, I. Yu. Morina, A.A. Perminova, A.O. Shpakov. Effect of Low-Molecular-Weight Allosteric Agonists of the Luteinizing Hormone Receptor on Its Expression and Distribution in Rat Testes. J Evol Biochem Phys. 2021, 57, 208–220. doi: 10.1134/S0022093021020034.
- Bakhtyukov A.A., Derkach K.V., Stepochkina A.M., Sorokoumov V.N., Bayunova L.V., Lebedev I.A., Shpakov A.O. / The effect of metformin therapy on luteinizing hormone receptor agonists-induced stimulation of testosterone production and spermatogenesis in diabetic rats. J Evol Biochem Phys. 2021, V. 57. № 6. P. 1382–1393. doi: 10.1134/S002209302106017X.
- 12. Derkach, K.V., Romanova, I.V., Bakhtyukov, A.A., Morina, I.Y., Dar’in, D.V., Sorokoumov, V.N., Shpakov, A.O. / The Effect of Low-Molecular-Weight Allosteric Agonist of Luteinizing Hormone Receptor on Functional State of the Testes in Aging and Diabetic Rats. Bulletin of Experimental Biology and Medicine, 2021,171 (1), pp. 81-86. doi: 10.1007/s10517-021-05177-5.
2020
- Mikhaylov, V. N.; Pavlov, A. O.; Ogorodnov, Y. V; Spiridonova, D. V; Sorokoumov, V. N.; Balova, I. A. N-Propargylation and Copper(I)-Catalyzed Azide-Alkyne Cycloaddition as a Convenient Strategy for Directed Post-Synthetic Modification of 4-Oxo-1,4-Dihydrocinnoline Derivatives. Chem. Heterocycl. Compd. 2020, 56 (7), 915–922, doi: 10.1007/s10593-020-02750-0.
- Danilkina, N. A., D’yachenko, A., Govdi, A. I., Khlebnikov, A. F., Kornyakov, I., Bräse, S., & Balova, I. A. (2020). Intramolecular Nicholas Reactions in the Synthesis of Heteroenediynes fused to Indole, Triazole and Isocoumarin. The Journal of Organic Chemistry, doi: 10.1021/acs.joc.0c00930.
- Danilkina, N. A.; Govdi, A. I.; Balova, I. A. 5-Iodo-1H-1,2,3-triazoles as Versatile Building Blocks. Synthesis (Stuttg). 2020, 11–16, doi: 10.1055/s-0039-1690858.
- Danilkina, N. A.; Vasileva, A. A.; Balova, I. A. A.E.Favorskii’s scientific legacy in modern organic chemistry: prototropic acetyleneallene isomerization and the acetylene zipper reaction. Russ. Chem. Rev. 2020, 89, 125–171, doi: 10.1070/rcr4902.
- Mikhaylov, V. N.; Sorokoumov, V. N.; Novikov, A. S.; Melnik, M. V; Tskhovrebov, A. G.; Balova, I. A. Intramolecular hydrogen bonding stabilizes trans-configuration in a mixed carbene/isocyanide PdII complexes. J. Organomet. Chem. 2020, 121174, doi: 10.1016/j.jorganchem.2020.121174.
- Gordeychuk, D. I.; Sorokoumov, V. N.; Mikhaylov, V. N.; Panov, M. S.; Khairullina, E. M.; Melnik, M. V.; Kochemirovsky, V. A.; Balova, I. A. Copper-Based Nanocatalysts Produced via Laser-Induced Ex Situ Generation for Homo- and Cross-Coupling Reactions. Chem. Eng. Sci. 2020, 227, 115940. doi: 10.1016/j.ces.2020.115940.
2019
- Tskhovrebov, A. G.; Novikov, A. S.; Odintsova, O. V.; Mikhaylov, V. N.; Sorokoumov, V. N.; Serebryanskaya, T. V.; Starova, G. L. Supramolecular polymers derived from the PtII and PdII schiff base complexes via C(sp2)–H … Hal hydrogen bonding: Combined experimental and theoretical study. J. Organomet. Chem. 2019, 886, 71–75, doi: 10.1016/j.jorganchem.2019.01.023.
- Danilkina, N. A.; Bukhtiiarova, N. S.; Govdi, A. I.; Vasileva, A. A.; Rumyantsev, A. M.; Volkov, A. A.; Sharaev, N. I.; Povolotskiy, A. V.; Boyarskaya, I. A.; Kornyakov, I. V.; Tokareva, P. V.; Balova, I. A. Synthesis and Properties of 6-Aryl-4-azidocinnolines and 6-Aryl-4-(1,2,3-1H-triazol-1-yl)cinnolines. Molecules 2019, 24, 2386, doi: 10.3390/molecules24132386.
- Danilkina, N.; Rumyantsev, A.; Lyapunova, A.; D’yachenko, A.; Khlebnikov, A.; Balova, I. 10-Membered Azaenediyne Fused to a Benzothiophene through the Nicholas Macrocyclization: Synthesis and DNA Cleavage Ability. Synlett 2019, 30, 161–166. DOI: 10.1055/s-0037-1610352.
- Govdi, A. I.; Danilkina, N. A.; Ponomarev, A. V.; Balova, I. A. 1-Iodobuta-1,3-diynes in Copper-Catalyzed Azide–Alkyne Cycloaddition: A One-Step Route to 4-Ethynyl-5-iodo-1,2,3-triazoles. J. Org. Chem. 2019, 84, 1925–1940. DOI: 10.1021/acs.joc.8b02916.
2018
- Kulyashova, A. E.; Ponomarev, A. V.; Selivanov, S. I.; Khlebnikov, A. F.; Popik, V. V.; Balova, I. A. Cr(II)-promoted internal cyclization of acyclic enediynes fused to benzo[b]thiophene core: Macrocycles versus 2-methylenecycloalkan-1-ols formation. Arab. J. Chem. 2018. DOI: 10.1016/j.arabjc.2018.05.005.
- Mikhaylov, V.; Sorokoumov, V.; Liakhov, D.; Tskhovrebov, A.; Balova, I. Polystyrene-Supported Acyclic Diaminocarbene Palladium Complexes in Sonogashira Cross-Coupling: Stability vs. Catalytic Activity. Catalysts 2018, 8, 141. DOI: 10.3390/catal8040141.
- Tskhovrebov, A. G.; Vasileva, A. A.; Goddard, R.; Riedel, T.; Dyson, P. J.; Mikhaylov, V. N.; Serebryanskaya, T. V.; Sorokoumov, V. N.; Haukka, M. Palladium(II)-Stabilized Pyridine-2-Diazotates: Synthesis, Structural Characterization, and Cytotoxicity Studies. Inorg. Chem. 2018, 57, 930–934. DOI: 10.1021/acs.inorgchem.8b00072.
- Zakharova, I. O.; Sorokoumov, V. N.; Bayunova, L. V; Derkach, K. V; Shpakov, A. O. 4-oxo-1,4-dihydrocinnoline Derivative with Phosphatase 1B Inhibitor Activity Enhances Leptin Signal Transduction in Hypothalamic Neurons. J. Evol. Biochem. Physiol. 2018, 54, 273–280. DOI: 10.1134/S0022093018040038.
- Konovalov, A. I.; Antipin, I. S.; Burilov, V. A.; Madzhidov, T. I.; Kurbangalieva, A. R.; Nemtarev, A. V.; Solovieva, S. E.; Stoikov, I. I.; Mamedov, V. A.; Zakharova, L. Y.; Gavrilova, E. L.; Sinyashin, O. G.; Balova, I. A.; Vasilyev, A. V.; Zenkevich, I. G.; Krasavin, M. Y.; Kuznetsov, M. A.; Molchanov, A. P.; Novikov, M. S.; Nikolaev, V. A.; Rodina, L. L.; Khlebnikov, A. F.; Beletskaya, I. P.; Vatsadze, S. Z.; Gromov, S. P.; Zyk, N. V.; Lebedev, A. T.; Lemenovskii, D. A.; Petrosyan, V. S.; Nenaidenko, V. G.; Negrebetskii, V. V.; Baukov, Y. I.; Shmigol’, T. A.; Korlyukov, A. A.; Tikhomirov, A. S.; Shchekotikhin, A. E.; Traven’, V. F.; Voskresenskii, L. G.; Zubkov, F. I.; Golubchikov, O. A.; Semeikin, A. S.; Berezin, D. B.; Stuzhin, P. A.; Filimonov, V. D.; Krasnokutskaya, E. A.; Fedorov, A. Y.; Nyuchev, A. V.; Orlov, V. Y.; Begunov, R. S.; Rusakov, A. I.; Kolobov, A. V.; Kofanov, E. R.; Fedotova, O. V.; Egorova, A. Y.; Charushin, V. N.; Chupakhin, O. N.; Klimochkin, Y. N.; Osyanin, V. A.; Reznikov, A. N.; Fisyuk, A. S.; Sagitullina, G. P.; Aksenov, A. V.; Aksenov, N. A.; Grachev, M. K.; Maslennikova, V. I.; Koroteev, M. P.; Brel’, A. K.; Lisina, S. V.; Medvedeva, S. M.; Shikhaliev, K. S.; Suboch, G. A.; Tovbis, M. S.; Mironovich, L. M.; Ivanov, S. M.; Kurbatov, S. V.; Kletskii, M. E.; Burov, O. N.; Kobrakov, K. I.; Kuznetsov, D. N. Modern Trends of Organic Chemistry in Russian Universities. Russ. J. Org. Chem. 2018, 54, 157–371. DOI: 10.1134/S107042801802001X.
- Lyapunova, A. G.; Danilkina, N. A.; Rumyantsev, A. M.; Khlebnikov, A. F.; Chislov, M. V.; Starova, G. L.; Sambuk, E. V.; Govdi, A. I.; Bräse, S.; Balova, I. A. Relative Reactivity of Benzothiophene-Fused Enediynes in the Bergman Cyclization. J. Org. Chem. 2018, 83, 2788–2801. DOI: 10.1021/acs.joc.7b03258.
2017
- Shpakova, E. A.; Sorokoumov, V. N.; Akent’ev, A. V.; Derkach, K. V.; Tennikova, T. B.; Shpakov, A. O. The relationship between micelle formation and biological activity of peptide 562–572 of luteinizing hormone receptor modified with decanoyl radicals. Cell tissue biol. 2017, 11, 227–233. DOI: 10.1134/S1990519X17030105.
- Sorokoumov, V. N.; Shpakov, A. O. Protein phosphotyrosine phosphatase 1B: Structure, function, role in the development of metabolic disorders and their correction by the enzyme inhibitors. J. Evol. Biochem. Physiol. 2017, 53, 259–270. DOI: 10.1134/S0022093017040020.
- Gordeychuk, D. Vladimir Kochemirovsky, Victor Sorokoumov, Ilya Tumkin, Alexey Kuzmin, Irina Balova. Copper Particles Generated During in situ Laser-induced Synthesis Exhibit Catalytic Activity Towards Formation of Gas Phase. J. Laser Micro/Nanoengineering 2017, 12, 57–61. DOI: 10.2961/jlmn.2017.02.0001.
- Mikhaylov, V. N.; Sorokoumov, V. N.; Balova, I. A. Polystyrene-supported diaminocarbene complexes of palladium(II): synthesis, characterization and application as a precatalyst in Sonogashira–Hagihara and Suzuki–Miyaura cross coupling. Russ. Chem. Rev. 2017, 86, 459–473. DOI: 10.1070/RCR4715.
- Lyapunova, A. G.; D’yachenko, A. S.; Danilkina, N. A. Potassium fluoride for one-pot desilylation and the Sonogashira coupling of ethynylsilanes and buta-1,3-diynylsilanes. Russ. J. Org. Chem. 2017, 53, 800–804. DOI: 10.1134/S1070428017050268.
- Govdi, A. I.; Kulyashova, A. E.; Vasilevsky, S. F.; Balova, I. A. Functionalized buta-1,3-diynyl-N-methylpyrazoles by sequential “diacetylene zipper” and Sonogashira coupling reactions. Tetrahedron Lett. 2017, 58, 762–765. DOI: 10.1016/j.tetlet.2017.01.032.
2016
- Danilkina, N. A.; Gurskaya, L. Y.; Vasilyev, A. V; Balova, I. A. Towards Isocoumarin-Fused Enediyne Systems through the Electrophilic Cyclization of Methyl o-(Buta-1,3-diynyl)benzoates. European J. Org. Chem. 2016, 739–747. DOI: 10.1002/ejoc.201501262.
- Lyapunova, A. G.; Danilkina, N. A.; Khlebnikov, A. F.; Köberle, B.; Bräse, S.; Balova, I. A. Oxaenediynes through the Nicholas-Type Macrocyclization Approach. European J. Org. Chem. 2016, 2016, 4842–4851. DOI: 10.1002/ejoc.201600767.
- Mikhaylov, V. N.; Sorokoumov, V. N.; Korvinson, K. A.; Novikov, A. S.; Balova, I. A. Synthesis and Simple Immobilization of Palladium(II) Acyclic Diaminocarbene Complexes on Polystyrene Support as Efficient Catalysts for Sonogashira and Suzuki–Miyaura Cross-Coupling. Organometallics 2016, 35, 1684–1697. DOI: 10.1021/acs.organomet.6b00144.
- Mikhailov, V. N.; Korvinson, K.; Sorokoumov, V. N. Chiral acyclic diaminocarbene complexes of palladium(II) immobilized on a polymeric support as promising catalysts of the Suzuki reaction. Russ. J. Gen. Chem. 2016, 86, 2473–2476. DOI: 10.1134/S1070363216110128.
2009–2015
- Danilkina, N. A.; Vlasov, P. S.; Vodianik, S. M.; Kruchinin, A. A.; Vlasov, Y. G.; Balova, I. A. Synthesis and chemosensing properties of cinnoline-containing poly(arylene ethynylene)s. Beilstein J. Org. Chem. 2015, 11, 373–384. DOI: 10.3762/bjoc.11.43.
- Danilkina, N. A.; Lyapunova, A. G.; Khlebnikov, A. F.; Starova, G. L.; Bräse, S.; Balova, I. A. Ring-Closing Metathesis of Co2(CO)6–Alkyne Complexes for the Synthesis of 11-Membered Dienediynes: Overcoming Thermodynamic Barriers. J. Org. Chem. 2015, 80, 5546–5555. DOI: 10.1021/acs.joc.5b00409.
- Kulyashova, A. E.; Mikheeva, E. V.; Danilkina, N. A.; Balova, I. A. Synthesis of 2-(buta-1,3-diynyl)-N,N-dimethylanilines Using Reductive Methylation Step. Mendeleev Commun. 2014, 24, 102–104. DOI: 10.1016/j.mencom.2014.03.013.
- Danilkina, N. A.; Kulyashova, A. E.; Khlebnikov, A. F.; Bräse, S.; Balova, I. A. Electrophilic Cyclization of Aryldiacetylenes in the Synthesis of Functionalized Enediynes Fused to a Heterocyclic Core. J. Org. Chem. 2014, 79, 9018–9045. DOI: 10.1021/jo501396s.
- Mikhailov, V. N.; Savicheva, E. A.; Sorokoumov, V. N.; Boyarskii, V. P. Catalytic activity of palladium(II) diaminocarbene complexes in the Sonogashira and Suzuki reactions. Russ. J. Org. Chem. 2013, 49, 551–554. DOI: 10.1134/S107042801304009X.
- Ryabukhin, D. S.; Sorokoumov, V. N.; Savicheva, E. A.; Boyarskiy, V. P.; Balova, I. A.; Vasilyev, A. V. Catalytic activity of palladium acyclic diaminocarbene complexes in the synthesis of 1,3-diarylpropynones via Sonogashira reaction: cross- versus homo-coupling. Tetrahedron Lett. 2013, 54, 2369–2372. DOI: 10.1016/j.tetlet.2013.02.086.
- Kulyashova, A. E.; Sorokoumov, V. N.; Popik, V. V; Balova, I. A. An acetylene zipper—Sonogashira reaction sequence for the efficient synthesis of conjugated arylalkadiynols. Tetrahedron Lett. 2013, 54, 2235–2238. DOI: 10.1016/j.tetlet.2013.02.066.
- Danilkina, N. A.; Kulyashova, A. E.; Balova, I. A. Intramolecular cyclizations of functionalized diynes. Chem. Heterocycl. Compd. 2012, 48, 95–106. DOI: 10.1007/s10593-012-0973-7.
- Danilkina, N.; Nieger, M.; Selivanov, S.; Bräse, S.; Balova, I. Electrophilic Cyclization and Ring-Closing Metathesis as Key Steps in the Synthesis of a 12-Membered Cyclic Enediyne. European J. Org. Chem. 2012, 2012, 5660–5664. DOI: 10.1002/ejoc.201200881.
- Danilkina, N. A.; Gorbunova, E. G.; Sorokoumov, V. N.; Balova, I. A. Study of cyclyzation of o-(1-Alkynyl)- and o-(1,3-Butadiynyl)aryltriazenes under the action of acids. Russ. J. Org. Chem. 2012, 48, 1424–1434. DOI: 10.1134/S1070428012110048.
- Danilkina, N. A.; Mikhaylov, L. E.; Ivin, B. A. Reaction of acetylenedicarboxylic acids esters with 4,5-dihydro-1H-pyrazole-1-carbothioamides and 3,4,5,6-tetrahydro-2H-1,2,4-triazepine-3-thiones. Chem. Heterocycl. Compd. 2011, 47, 886–900. DOI: 10.1007/s10593-011-0850-9.
- Novikov, R. V.; Danilkina, N. A.; Balova, I. A. Cyclocondensation of n-(prop-2-yn-1-yl)- and n-(penta-2,4-diyn-1-yl)- o-phenylenediamines with phenyl isothiocyanate and carbon disulfide. Chem. Heterocycl. Compd. 2011, 47, 758–766. DOI: 10.1007/s10593-011-0831-z.
- Sorokoumov, V. N.; Popik, V. V.; Balova, I. A. Access to 2,3-bis(buta-1,3-diynyl)pyridines. Mendeleev Commun. 2011, 21, 19–20. DOI: 10.1016/j.mencom.2011.01.008.
- Tskhovrebov, A. G.; Luzyanin, K. V.; Kuznetsov, M. L.; Sorokoumov, V. N.; Balova, I. A.; Haukka, M.; Kukushkin, V. Y. Substituent R-Dependent Regioselectivity Switch in Nucleophilic Addition of N-Phenylbenzamidine to PdII- and PtII-Complexed Isonitrile RN≡C Giving Aminocarbene-Like Species. Organometallics 2011, 30, 863–874. DOI: 10.1021/om101041g.
- Danilkina, N.; Bräse, S.; Balova, I. Electrophilic Cyclization of Buta-1,3-diynylarenes: Synthesis of Precursors of (Z)-3-Ene-1,5-diyne Systems Fused to Heterocycles. Synlett 2011, 2011, 517–520. DOI: 10.1055/s-0030-1259547.
- Vinogradova, O. V.; Sorokoumov, V. N.; Balova, I. A. A short route to 3-alkynyl-4-bromo(chloro)cinnolines by Richter-type cyclization of ortho-(dodeca-1,3-diynyl)aryltriaz-1-enes. Tetrahedron Lett. 2009, 50, 6358–6360. DOI: 10.1016/j.tetlet.2009.08.103.

