Department of Organic Chemistry
Research Group of Professor Irina Balova
Short URL for media go.spbu.ru/rgbalova
- Acetylenic and diacetylenic compounds in organic synthesis
- Synthesis of heterocycles and ethynylheterocycles
- Synthesis of acyclic and cyclic enediynes
- New catalysts for the synthesis of acetylenic compounds
- Flow chemistry and microreactor synthesis
- Development of new protein tyrosine phosphatase 1B inhibitors based on 4-oxo-1,4-dihydrocinnoline derivatives

Group Members
 |
Group Leader Balova Irina A.Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Danilkina Natalia A.Ph.D., Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Efremova MariyaPh.D., Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Govdi Anastasia I.Ph.D., Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Sorokoumov Viktor N.Ph.D., Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Kaminsky NikitaMSc, Research Engineer This email address is being protected from spambots. You need JavaScript enabled to view it. |
Students
 |
Khmelevskaya Ekaterinapostgraduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Vidyakina Aleksandrapostgraduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Didenko Egor2 year graduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Kim Mia1 year graduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Ogurtsova Anna1 year graduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Korolyov-Zelyoniy Kirill4 year undergraduate student |
 |
Kutuzov Yaroslav4 year undergraduate student |
 |
Galkin Egor3 year undergraduate student |
 |
Hashimova Diana2 year undergraduate student |
 |
Kryukova Polina2 year undergraduate student |
 |
Menchikov Vasiliy2 year undergraduate student |
 |
Vlasova Elena2 year undergraduate student |
 |
Votchel Elena2 year undergraduate student |
 |
Parfenyuk Anna2 year undergraduate student |
 |
Melnikov Vladimir1 year undergraduate student |
Research Topic 1
Acetylene compounds in organic synthesis and for the construction of compounds with useful properties
(A) Synthetic approaches toward functionalized diacetylenes
Among different known synthetic routes toward terminal diynes «diacetylene zipper» reaction, which has been discovered in our research group, is of special importance. [A] It is a unique process of simultaneous migration of two conjugated triple bonds from an internal to a terminal position promoted by lithium 2-aminoethanamide. The scope of the reaction is unfunctionalized alkadiynes along with diacetylenic alcohols. [B][1]
«Diacetylene zipper» reaction followed by functionalization of lithium acetylides was used in the synthesis of diacetylenic alcohols [C][D], ketones, [2] carboxylic acids, [D] tetraynes [E] and other derivatives.
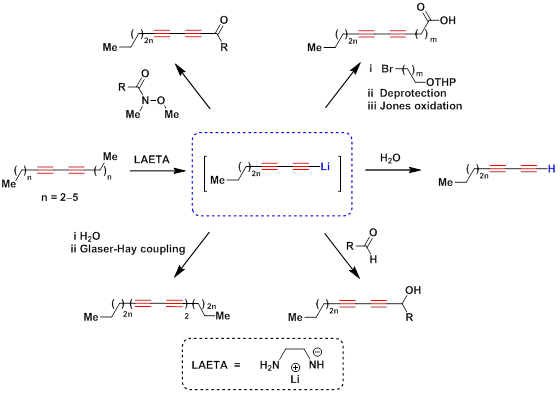
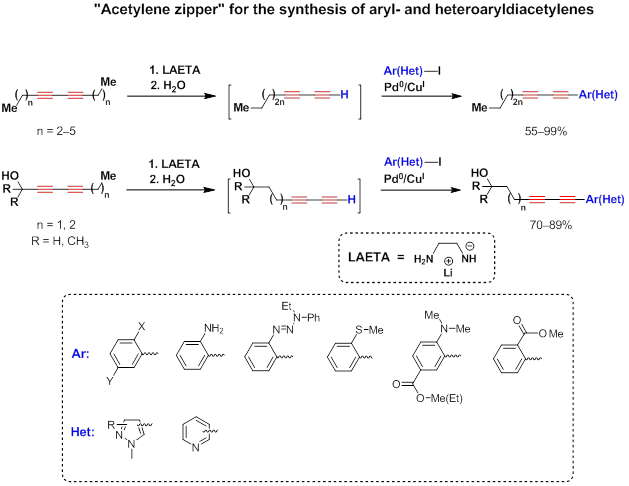
Being combined with the Sonogashira coupling, «diacetylene zipper» reaction was used as a general-purpose method for the synthesis of aryl- and heteroaryldiacetylenes. [3][4][5][6][1][7][8]
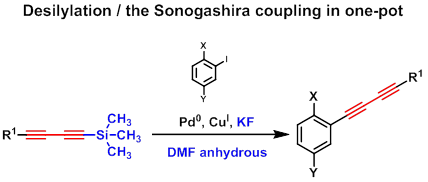
Removing of protecting groups is known to be the second route toward terminal diacetylenic compounds. Trimethylsilyl (TMS) group can be deprotected under mild conditions. Therefore desilylation of TMS-diacetylenes using potassium fluoride (KF) and the Sonogashira coupling being held in one-pot was found to be very convenient for the synthesis of functionalized arenes bearing buta-1,3-diynyl moiety. [9]
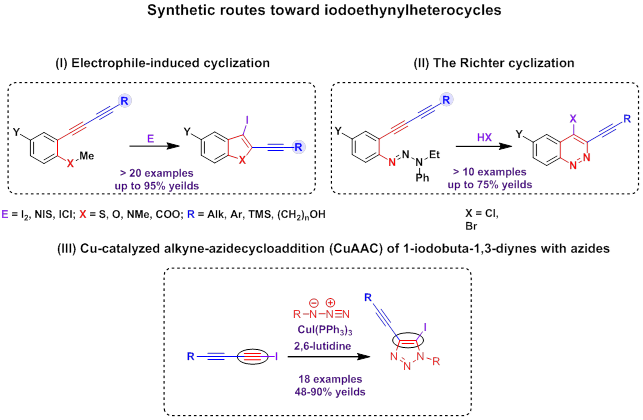
(B) Diacetylenic compounds in the synthesis of heterocycles
Two conjugated triple bonds are endowed with a unique synthetic potential. The research in our group revealed that aryldiacetylenes with a heteroatom functional group at ortho-position with two conjugated triple bonds are able to undergo heterocyclization (electrophile-induced cyclization, [10][11][12] and the Richter-type cyclization [5][13][14]) through only one of two triple bonds, remaining the second one untouched. These transformations afford heterocycles with a triple bond and a halogen atom at neighboring С atoms.
The method described was used for the synthesis of iodoethynylbenzothiophenes, -benzofurans, -indoles, -isocoumarines and 4-bromo(chloro)cinnolines.
Recently we have also discovered that Cu-catalyzed azide-alkyne cycloaddition (CuAAC) of 1-iodobuta-1,3-diynes with organic azides is a high-yielded and functional group tolerant synthetic method for the preparation of 5-iodo-4-ethynyl-1H-[1,2,3]triazoles. [15]
Both functional groups (a triple bond and a halogen atom) are of special importance for further functionalization of a heterocycle formed. Based on synthetic techniques for the synthesis of iodoethynylheterocycles we have elaborated a three-step synthetic route toward heterocycle-fused analogues of naturally-occurring enediyne antibiotics.
(C) Synthesis and properties of enediyne antibiotics analogues
Enediyne antibiotics are compounds with a high level of anticancer and antibacterial activity caused by a special structural feature – (Z)-hexa-3-en-1,5-diyne system. Being incorporated into a 9- or 10-membered cycle, an enediyne moiety is able to undergo the Bergman cyclization at ambient temperatures with the formation of highly reactive 1,4-phenylene diradicals which can damage DNA.
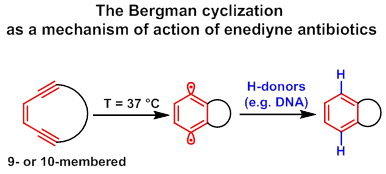
We focus our research on the search of simple synthetic analogues of enediyne antibiotics among enediyne systems fused to a heterocycle. The synthetic approach toward iodoethynylheterocycles in cooperation with the Sonogashira coupling allowed acetylene moieties with specially functional groups to be introduced into a heterocycle formed at neighboring C atoms. In turn, it provides an opportunity for the synthesis of special starting acyclic enediyne compounds for the next macrocyclization step. Different synthetic macrocyclization tools for the synthesis of cyclic enediynes have been tested in our group: the Nozaki-Hiyama-Kishi reaction, [16][17] ring-closing metathesis (RCM) [11][18] and the Nicholas reaction. [19][20][21]
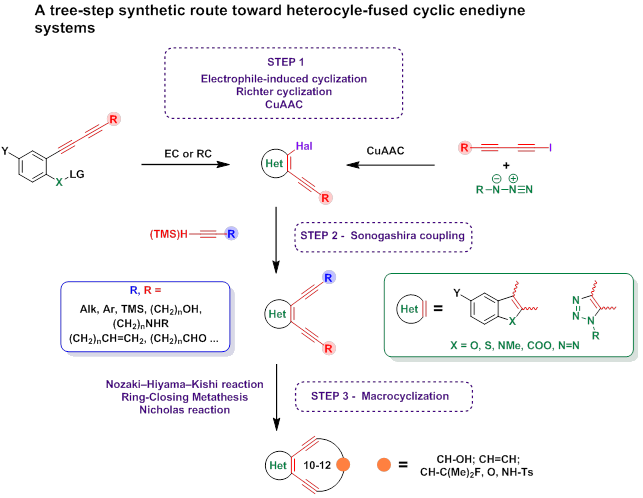
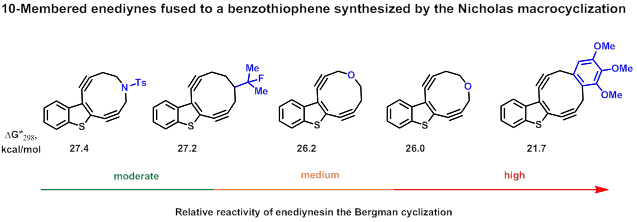
The Nicholas reaction as a macrocyclization tool was found to be the most promising and universal technique for these purposes. Thus several 10-membered enediynes fused to a benzothiophene have been synthesized and used for the creation and verification of the scale of relative reactivity of 10-membered enediynes. [20]
In collaboration with the Department of Genetics and Biotechnology of SPbU we have estimated that 10-membered benzothiophene-fused enediynes are able to cleave plasmid DNA inducing both single and double strand breaks. This property of enediynes is of great importance for the search of new anticancer compounds within benzothiophene-fused enediyne family.
Today we are working at the synthesis of small library of 10-membered enediynes fused to other heterocycles.
(D) Synthesis of new fluorescent poly(arylene ethynylene)s
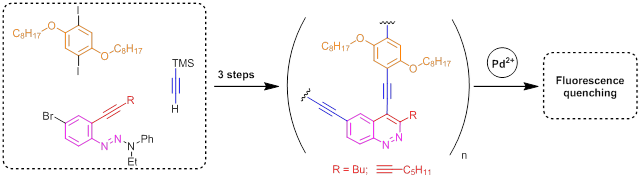
Nowadays poly(arylene ethynylene)s represents a promising type of compounds for the construction of polymers with precise desired properties. Thus for poly(arylene ethynylene)s bearing special functional groups responsible for an analyte binding, a fluorescence quenching effect can be detected as a response for the interaction with the analyte molecule. Such polymers are of great interest for the creation of new detecting systems. We have synthesized poly(arylene ethynylene)s with cinnoline rings in a polymer chain. The fluorescence quenching effect was detected as a special response for PdII ions.[22]
References
- L. A. Remizova, I. A. Balova, and I. A. Favorskaya, Prototropic isomerization of diacetylenic compounds. Zh. Org. Khim., 1986, 22, 2459 (in Russian).
- I. A. Balova, I. V. Zakharova, and L. A. Remizova. Studies on the Prototropic Isomerization of Diacetylene Alcohols. Russ. J. Org. Chem. (Engl. Transl.) 1993, 29, 1439.
- Balova, I. A.; Morozkina, S. N.; Voskresenskii, S. V.; Remizova, L. A. Consecutive reactions of diacetylenes: "Acetylene zipper" reaction and hydroxyalkylation of lithium 1,3-alkadiynides as a synthetic route to α- and β-long-chain diacetylenic alcohols. Russ. J. Org. Chem. (Engl. Transl.) 2000, 36 (10), 1428.
- Balova, I. A.; Remizova, L. A.; Makarycheva, V. F.; Rumyantseva, E. G.; Favorskaya, I. A. Russ. J. Org. Chem. (Engl. Transl.) 1991, 27, 55.
- Balova, I. A., Remizova, L.A. «The "Acetylene Zipper" Reaction of Diacetylene Compounds in the Synthesis of Tetraynes» Russ. J. Org. Chem. (Engl. Transl.) 1994, 30, (2) 213.
- Kulyashova, A.E.; Sorokoumov, V.N.; Popik, V. V; Balova, I.A. An acetylene zipper—Sonogashira reaction sequence for the efficient synthesis of conjugated arylalkadiynols. Tetrahedron Lett. 2013, 54, 2235–2238, https://linkinghub.elsevier.com/retrieve/pii/S004040391300316X
- Novikov, R. V; Vasil’ev, A.A.; Balova, I.A. Convenient synthesis of dodeca-1,3-diynyl ketones by the “diacetylene zipper” reaction. Russ. Chem. Bull. 2005, 54, 1043–1045, http://link.springer.com/10.1007/s11172-005-0355-8
- Balova, I.A.; Morozkina, S.N.; Knight, D.W.; Vasilevsky, S.F. A one-pot synthesis of 1-arylalka-1,3-diynes by sequential acetylene zipper and Sonogashira reactions. Tetrahedron Lett. 2003, 44, 107–109, http://linkinghub.elsevier.com/retrieve/pii/S0040403902024966
- Balova, I.A.; Sorokoumov, V.N.; Morozkina, S.N.; Vinogradova, O. V; Knight, D.W.; Vasilevsky, S.F. A Convenient Synthesis of Functionalised 1-Aryl-1,3-alkadiynes. Eur. J. Org. Chem. 2005, 2005, 882–888, http://doi.wiley.com/10.1002/ejoc.200400688
- Vinogradova, O. V.; Sorokoumov, V.N.; Balova, I.A. A short route to 3-alkynyl-4-bromo(chloro)cinnolines by Richter-type cyclization of ortho-(dodeca-1,3-diynyl)aryltriaz-1-enes. Tetrahedron Lett. 2009, 50, 6358–6360, http://dx.doi.org/10.1016/j.tetlet.2009.08.103
- Sorokoumov, V.N.; Popik, V. V.; Balova, I.A. Access to 2,3-bis(buta-1,3-diynyl)pyridines. Mendeleev Commun. 2011, 21, 19–20, https://linkinghub.elsevier.com/retrieve/pii/S0959943611000095
- Govdi, A.I.; Kulyashova, A.E.; Vasilevsky, S.F.; Balova, I.A. Functionalized buta-1,3-diynyl- N -methylpyrazoles by sequential “diacetylene zipper” and Sonogashira coupling reactions. Tetrahedron Lett. 2017, 58, 762–765, http://dx.doi.org/10.1016/j.tetlet.2017.01.032
- Kulyashova, A.E.; Mikheeva, E. V.; Danilkina, N.A.; Balova, I.A. Synthesis of 2-(buta-1,3-diynyl)-N,N-dimethylanilines Using Reductive Methylation Step. Mendeleev Commun. 2014, 24, 102–104, http://dx.doi.org/10.1016/j.mencom.2014.03.013
- Lyapunova, A.G.; D’yachenko, A.S.; Danilkina, N.A. Potassium fluoride for one-pot desilylation and the Sonogashira coupling of ethynylsilanes and buta-1,3-diynylsilanes. Russ. J. Org. Chem. 2017, 53, 800–804, http://link.springer.com/10.1134/S1070428017050268
- Danilkina, N.; Bräse, S.; Balova, I. Electrophilic Cyclization of Buta-1,3-diynylarenes: Synthesis of Precursors of (Z)-3-Ene-1,5-diyne Systems Fused to Heterocycles. Synlett 2011, 2011, 517–520, http://www.thieme-connect.de/DOI/DOI?10.1055/s-0030-1259547
- Danilkina, N.A.; Kulyashova, A.E.; Khlebnikov, A.F.; Bräse, S.; Balova, I.A. Electrophilic Cyclization of Aryldiacetylenes in the Synthesis of Functionalized Enediynes Fused to a Heterocyclic Core. J. Org. Chem. 2014, 79, 9018–9045, http://pubs.acs.org/doi/10.1021/jo501396s
- Danilkina, N.A.; Gurskaya, L.Y.; Vasilyev, A. V.; Balova, I.A. Towards Isocoumarin-Fused Enediyne Systems through the Electrophilic Cyclization of Methyl o -(Buta-1,3-diynyl)benzoates. Eur. J. Org. Chem. 2016, 2016, 739–747, http://doi.wiley.com/10.1002/ejoc.201501262
- Vinogradova, O. V.; Sorokoumov, V.N.; Vasilevsky, S.F.; Balova, I.A. The Richter reaction of ortho-(alka-1,3-diynyl)aryldiazonium salts. Tetrahedron Lett. 2007, 48, 4907–4909, https://linkinghub.elsevier.com/retrieve/pii/S0040403907009203
- Vinogradova, O. V.; Sorokoumov, V.N.; Vasilevskii, S.F.; Balova, I.A. Studies on cyclization of o-(alka-1,3-diynyl)arenediazonium salts. Russ. Chem. Bull. 2008, 57, 1725–1733, http://link.springer.com/article/10.1007/s11172-008-0228-z
- Govdi, A.I.; Danilkina, N.A.; Ponomarev, A. V.; Balova, I.A. 1-Iodobuta-1,3-diynes in Copper-Catalyzed Azide–Alkyne Cycloaddition: A One-Step Route to 4-Ethynyl-5-iodo-1,2,3-triazoles. J. Org. Chem. 2019, 84, 1925–1940, http://pubs.acs.org/doi/10.1021/acs.joc.8b02916
- Kulyashova, A.E.; Ponomarev, A.V.; Selivanov, S.I.; Khlebnikov, A.F.; Popik, V.V.; Balova, I.A. Cr(II)-promoted internal cyclization of acyclic enediynes fused to benzo[ b ]thiophene core: Macrocycles versus 2-methylenecycloalkan-1-ols formation. Arab. J. Chem. 2018, https://doi.org/10.1016/j.arabjc.2018.05.005
- Vinogradova, O. V.; Balova, I.A.; Popik, V. V. Synthesis and Reactivity of Cinnoline-Fused Cyclic Enediyne. J. Org. Chem. 2011, 76, 6937–6941, http://pubs.acs.org/doi/abs/10.1021/jo201148h
- Danilkina, N.; Nieger, M.; Selivanov, S.; Bräse, S.; Balova, I. Electrophilic Cyclization and Ring-Closing Metathesis as Key Steps in the Synthesis of a 12-Membered Cyclic Enediyne. Eur. J. Org. Chem. 2012, 2012, 5660–5664, http://doi.wiley.com/10.1002/ejoc.201200881
- Lyapunova, A.G.; Danilkina, N.A.; Khlebnikov, A.F.; Köberle, B.; Bräse, S.; Balova, I.A. Oxaenediynes through the Nicholas-Type Macrocyclization Approach. Eur. J. Org. Chem. 2016, 2016, 4842–4851, http://doi.wiley.com/10.1002/ejoc.201600767
- Lyapunova, A.G.; Danilkina, N.A.; Rumyantsev, A.M.; Khlebnikov, A.F.; Chislov, M. V.; Starova, G.L.; Sambuk, E. V.; Govdi, A.I.; Bräse, S.; Balova, I.A. Relative Reactivity of Benzothiophene-Fused Enediynes in the Bergman Cyclization. J. Org. Chem. 2018, 83, 2788–2801, http://pubs.acs.org/doi/10.1021/acs.joc.7b03258
- Danilkina, N.; Rumyantsev, A.; Lyapunova, A.; D’yachenko, A.; Khlebnikov, A.; Balova, I. 10-Membered Azaenediyne Fused to a Benzothiophene through the Nicholas Macrocyclization: Synthesis and DNA Cleavage Ability. Synlett 2019, 30, 161–166, http://www.thieme-connect.de/DOI/DOI?10.1055/s-0037-1610352
- Danilkina, N.A.; Vlasov, P.S.; Vodianik, S.M.; Kruchinin, A.A.; Vlasov, Y.G.; Balova, I.A. Synthesis and chemosensing properties of cinnoline-containing poly(arylene ethynylene)s. Beilstein J. Org. Chem. 2015, 11, 373–384, http://www.beilstein-journals.org/bjoc/content/11/1/43
Research Topic 2
The new catalysts and their application in the synthesis of acetylenic compounds
For the effective implementation of "atom-economy" transformations in the creation of new carbon-carbon and carbon-heteroatom bonds within acetylene derivatives and other organic compounds, our group conduct research aimed on the development of effective homogeneous and heterogeneous catalytic systems.
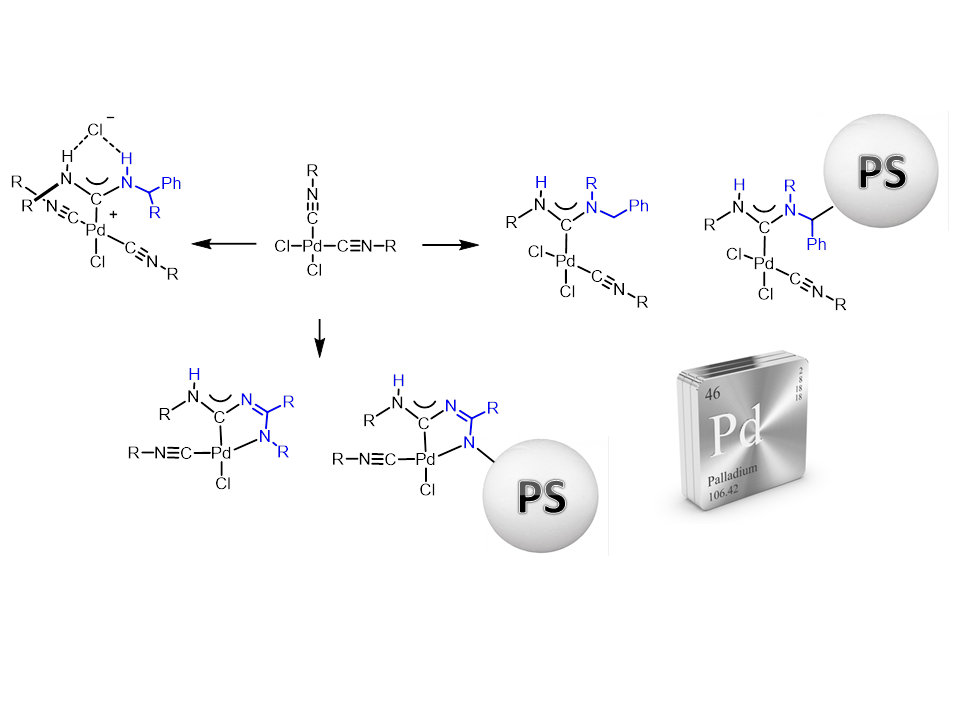
We can separated our work into two interdependent directions:
- The investigation of highly-active homogeneous catalysts based on palladium diaminocarbene complexes which are capable to work effectively under low catalytic concentrations conditions;
- The development of the reusable catalysts by immobilization of highly active homogeneous palladium complexes on a suitable support, which is inert under the reaction conditions used.
Currently, a method for the efficient heterogeneous catalytic systems preparation based on acyclic palladium diaminocarbene complexes has been developed using the metal-promoted reaction of nucleophilic addition of amino- and amidine- groups to isocyanides in palladium(II) complexes. The catalyst has high activity for the Sonogashira and Suzuki reactions on a wide set of substrates with the possibility of multiple use without significant loss of catalytic activity.
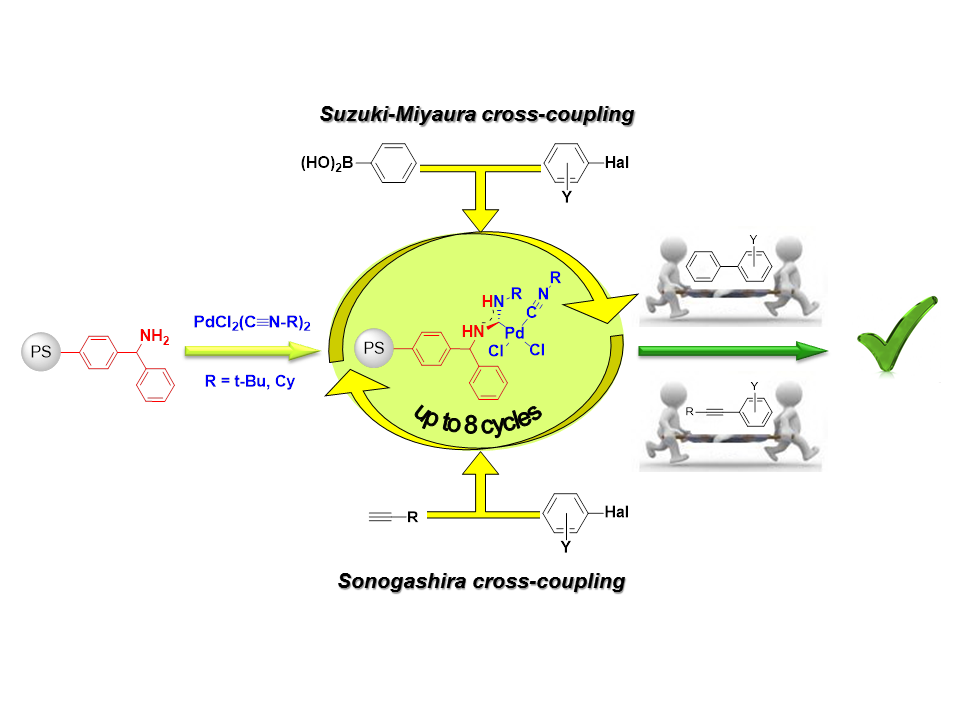
We have shown that the main contribution to the catalytic activity of heterogeneous precatalysts based on palladium acyclic diaminocarbene complexes is made by the palladium reversibly leached into the solution from the support during the reaction due to a new pathway of Pd(II) – Pd(0) activation through the carbodiimide formation. Our findings show the principally new route for Pd0-species generation and stabilization, and casts light on the nature of the catalysis for Pd(II)-complexes with different diaminocarbene ligand.
Finally, the ease of carbodiimide and HCl elimination from the certain ADCs-Pd with the formation of Pd(0) can be utilized for the catalyst activation in the manner used for commercially available Buchwald precatalysts.
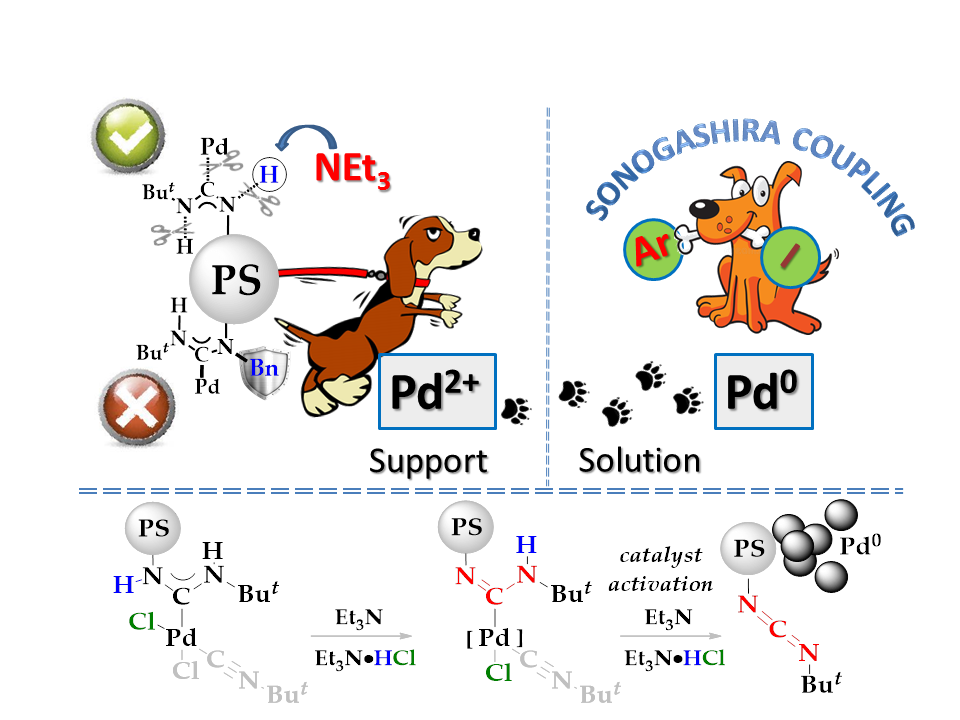
Our research team is also developing advanced catalytic systems which can catalyze low-reactive commercially important aryl chlorides under mild conditions (at room temperature without using strong bases).
Research Topic 3
The development of the new inhibitors of protein phosphotyrosine phosphatase 1B (PTP1B)
The enzyme protein phosphotyrosine phosphatase 1B (PTP1B) is the most important negative regulator of the insulin and leptin systems functioning through the activation of a cascade of tyrosine phosphorylation. The increased activity of PTP1B leads to the insulin (IR) and leptin resistances (LR) and is the cause of obesity, diabetes mellitus and other socially significant metabolic disorders. In this regard, an actual problem of modern medicine are deciphering the mechanisms of action of PTP1B and developing its selective inhibitors, which can allow treating the metabolic disorders associated with IR and LR. The most promising is the creation of "binary" PTP1B inhibitors that simultaneously interact with the catalytic (low-specific) and allosteric (high-specific) sites of PTP1B, which ensures the high efficiency and selectivity of their action.
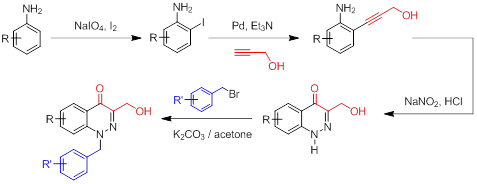
We proposed to use derivatives of 4-oxo-1,4-dihydrocinnoline as specific inhibitors of PTP1B. The developed approach to the synthesis of 4-oxo-1,4-dihydroindolone using Sonogashira reaction to obtain ortho-ethynylsubstituted anilines and the Richter reaction for the formation of cinnoline core, allows obtaining of 4-oxo-1,4-dihydrocinnoline derivatives in good yields with a small number of steps. Were also obtained promising results about the effectiveness of the synthesized derivative of 4-oxo-1,4-dihydrocinnoline – ethyl ester 3-(hydroximethyl)-4-oxo-1,4-dihydrcinnoline-6-carboxylic acid as a specific inhibitor PTP1B in vitro and in vivo. It was shown that the treatment of the primary culture of rat hypothalamic neurons with ethyl 3-(hydroxymethyl)-4-oxo-1,4-dihydrocinnoline-6-carboxylate increases the stimulating effect of leptin on phosphorylation of Akt-kinase and STAT3-protein, which indicates the suppression of the activity of PTP1B, which negatively regulates the effects of leptin. Along with this, this derivative of 4-oxo-1,4-dihydrocinoline when administered to rats caused a decrease in appetite, body weight, adipose tissue, improved metabolic parameters and increased insulin sensitivity in rats with an experimental model of obesity.
Publications
2024
- A. A. Vidyakina, S. A. Silonov, A. I. Govdi, A.Yu. Ivanov, E.P. Podolskaya, I. A. Balova, S. Bräse, N. A. Danilkina. Key role of cycloalkyne nature in alkyne-dye reagents for enhanced specificity of intracellular imaging by bioorthogonal bioconjugation. Org. Biomol. Chem., 2024, 22, 7637–7642, https://doi.org/10.1039/D4OB01032A
- A. V. Ponomarev, N. A. Danilkina, J. S. Okuneva, A. A. Vidyakina, E. A. Khmelevskaya, A. S. Bunev, A. M. Rumyantsev, A. I Govdi, T. Suarez, I. V. Alabugin, I. A Balova. Facile synthesis of diiodoheteroindenes and understanding their Sonogashira cross-coupling selectivity for the construction of unsymmetrical enediynes. Org. Biomol. Chem., 2024, 22, 4096–4107, https://doi.org/10.1039/D4OB00530A
- M. A. Gureev, N. A. Danilkina, A. F. Khlebnikov, I. A. Balova. Docking and Molecular Dynamics Studies on DNA-Heterocyclic Enediynes Interaction to Identify the Preferred Binding Mode. Russ. J. Gen. Chem. 2024, 94, 100–119, https://doi.org/10.1134/S1070363224140111
- I.A. Balova, N. A. Danilkina, A. I. Govdi. The chemistry of heterocycles in the 21st century (глава в обзоре). Russian Chemical Reviews, 2024, 93, RCR5125. DOI: 10.59761/RCR5125
- Cu-catalyzed cycloaddition of aryl azides to 1-iodobuta-1,3-diynes: an experimental and quantum chemical study of unusual regiochemistry. A. I. Govdi, N. A. Danilkina, A. A. Shtyrov, M. N. Ryazantsev, M. D. Kim, M. A. Kryukova, I. A. Balova. New J. Chem., 2024, 48, 4831-4845, https://doi.org/10.1039/D3NJ03823H
- Derkach, K.V., Sorokoumov, V.N., Morina, I.Y. et al. Regulatory Effects of 5-Day Oral and Intraperitoneal Administration of a Thienopyrimidine Derivative on the Thyroid Status in Rats. Bull Exp Biol Med, 177, 559–563 (2024). doi: 10.1007/s10517-024-06223-8
- Derkach K.V., Bakhtyukov A.A., Sorokoumov V.N., Didenko E.A., Romanova I.V., Morina I.Y., Lebedev I.A., Bayunova L.V., Shpakov A.O. Dynamics of gonadotropin and thienopyrimidine derivative TP03 effects on ovulation and ovarian steroidogenesis in Follimag-stimulated immature female rats // Reviews on Clinical Pharmacology and Drug Therapy. - 2024. - Vol. 22. - N. 1. - P. 53–65. doi: 10.17816/RCF622883
- Derkach, K.V., Bakhtyukov, A.A., Sorokoumov, V.N. et al. Low Molecular Weight Thyrotropin Receptor Inverse Agonist Is Active upon Both Intraperitoneal and Oral Administration. J Evol Biochem Phys, 60, 295–305 (2024). doi: 10.1134/S0022093024010216
- Meshalkin S.A. , Tsybulin S.V., Bardakov V.G., Tatarinov I.A., Shitov D.A., Tupikina E.Y., Efremova M.M., Antonov A.S. “Buttressing Effect” in the Halogen-Lithium Exchange in ortho-Bromo-N,N-dimethylanilines and Related Naphthalenes, Chem. Eur. J. 2024, e202303956. DOI: 10.1002/chem.202303956
- Efremova M.M., Ivanov A.V., Panikorovskii T.L., Molchanov A.P. Reactions of 1-Vinyl-4,5-dihydro-1H-benzo[g]indole with Nitrones in Presence of Nickel(II) Perchlorate, Russ. J. Gen. Chem., 2024, 94, S53–S59. DOI: 10.1134/S1070363224140081
- Efremova a M.M., Khashimova D.D., Kalinin N.S., Shtyrov A.A., Ryazantsev M.N., Spiridonova D.V., Govdi A.I., Balova I.A. Microwave assisted cycloaddition of benzonitrile oxides to 1-iodobuta-1,3-diynes, Mendeleev Communications, 2024, 34, 536–539. DOI: 10.1016/j.mencom.2024.06.022
2023
- A.A. Vidyakina, A. A. Shtyrov, M.N. Ryazantsev, A. F. Khlebnikov, I. E. Kolesnikov, V. V. Sharoyko. D. V. Spiridonova, I. A. Balova, S. Bräse, N. A. Danilkina. Development of Fluorescent Isocoumarin-Fused Oxacyclononyne – 1,2,3-Triazole Pairs. Chemistry — A European Journal, 2023, e202300540. Статья находится в открытом доступе и попала в Hot Topic: Click Chemistry издательства Wiley: 10.1002/(ISSN)1521-3773.hottopic-clickchemistry.
- A. A. Babushkina, V. N. Mikhailov. A. D. Ogurtsova, A. S. Bunev, V.N. Sorokoumov, I. A. Balova. The Richter reaction in the synthesis of combretastatin analogs. Russian Chemical Bulletin. 2023, 72(4), 1012-1022. DOI: 10.1007/s11172-023-3866-3.
- A. I. Govdi, S. O. Anisimov, N. A. Danilkina, A. S. Bunev. I. A. Balova. Acyclic enediynes fused to triazoleand benzothiophene containing propargylamine moieties. Mendeleev Commun., 2023, 33, 328–330. DOI: 10.1016/j.mencom.2023.04.010.
- K. V. Derkach, M. A. Gureev, A. A. Babushkina, V. N. Mikhaylov , I. O. Zakharova, A. A. Bakhtyukov, V. N. Sorokoumov, A. S. Novikov, M. Krasavin, A. O. Shpakov, I. A. Balova. Dual PTP1B/TC-PTP Inhibitors: Biological Evaluation of 3-(Hydroxymethyl)cinnoline-4(1H)-Ones, Int. J. Mol. Sci. 2023, 24(5), 4498; DOI: 10.3390/ijms24054498.
- Bakhtyukov A.A., Derkach K.V., Fokina E.A. , Lebedev I.A., Sorokoumov V.N., Bayunova, L.V., Shpakov A.O., / Effect of Different Luteinizing Hormone Receptor Agonists on Ovarian Steroidogenesis in Mature Female Rats. / J Evol Biochem Phys 59, 57–68 (2023). DOI: 10.1134/S0022093023010052.
- Fokina E.A., Derkach K.V., Bakhtuykov A.A., Sorokoumov V.N., Lebedev I.A., Morina I.Y., Shpakov A.O. / Stimulation Of Ovulation In Immature Female Rats Using Orthosteric And Allosteric Luteinizing Hormone Receptor Agonists / Doklady Rossijskoj akademii nauk. Nauki o žizni. – (2023). - Vol. 508. - N. 1. - P. 30-34. doi: 10.31857/S2686738922700032.
- Efremova M.М., Rumyantsev A.M., Babitova E.S., Ianshina T. M., Govd A.I. Synthesis of 5-ethynylisoxazoles based on 1,3-dipolar cycloaddition reactions of nitrile oxides with conjugated diynes. Russ. Chem. Bull. 2023, 72, 1717–1721. DOI: 10.1007/s11172-023-3952-5.
- T. Ianshina; A. Sidorin; K. Petrova; M. Shubert, A. Makeeva, E. Sambuk; A. Govdi, A. Rumyantsev, M. Padkina Effect of Methionineon Gene Expression in Komagataella phaffii Cells. Microorganisms. 2023, 11, 877. DOI: 10.3390/microorganisms11040877.
- S.G.Zlotin, K.S.Egorova, V.P.Ananikov, A.A.Akulov, M.V.Varaksin, O.N.Chupakhin, V.N.Charushin...., I.A.Balova, V.N.Sorokoumov et al. Russ. Chem. Rev., 2023, 92 (12) RCR5104.
- Derkach, K.V.; Lebedev, I.A.; Morina, I.Y.; Bakhtyukov, A.A.; Pechalnova, A.S.; Sorokoumov, V.N.; Kuznetsova, V.S.; Romanova, I.V.; Shpakov, A.O., Int. J. Mol. Sci., 24 (2023), 16618.
2022
- Danilkina, N.A.; Khmelevskaya, E.A.; Lyapunova, A.G.; D’yachenko, A.S.; Bunev, A.S.; Gasanov, R.E.; Gureev, M.A.; Balova, I.A. Functionalized 10-Membered Aza- and Oxaenediynes through the Nicholas Reaction. Molecules 2022, 27, 6071, doi: 10.3390/molecules27186071. Impact Factor: 4.927.
- Babushkina, A.A.; Mikhaylov, V.N.; Novikov, A.S.; Sorokoumov, V.N.; Gureev, M.A.; Kryukova, M.A.; Shpakov, A.O.; Balova, I.A. Synthesis , X-ray and DFT studies of 6-halo-3-(hydroxymethyl ) cinnolin-4(1H)-ones. Chem. Heterocycl. Compd. 2022, 58, 432–437, doi: 10.1007/s10593-022-03109-3. Impact Factor: 1.490.
- Govdi, A.I.; Tokareva, P.V.; Rumyantsev, A.M.; Panov, M.S.; Stellmacher, J.; Alexiev, U.; Danilkina, N.A.; Balova, I.A. 4,5-Bis(arylethynyl)-1,2,3-triazoles—A New Class of Fluorescent Labels: Synthesis and Applications. Molecules 2022, 27, doi: 10.3390/molecules27103191. Impact Factor: 4.927.
- Gholinejad, M.; Shojafar, M.; Sansano, J.M.; Mikhaylov, V.N.; Balova, I.A.; Khezri, R. Hyperbranched polymer immobilized palladium nanoparticles as an efficient and reusable catalyst for cyanation of aryl halides and reduction of nitroarenes. J. Organomet. Chem. 2022, 970–971, doi: 10.1016/j.jorganchem.2022.122359. Impact Factor: 2.345.
- Derkach, K. V; Fokina, E.A.; Bakhtyukov, A.A.; Sorokoumov, V.N.; Stepochkina, A.M.; Zakharova, I.O.; Shpakov, A.O. The Study of Biological Activity of a New Thieno[2,3-D]-Pyrimidine-Based Neutral Antagonist of Thyrotropin Receptor. Bull. Exp. Biol. Med. 2022, 172, 713–717, doi: 10.1007/s10517-022-05462-x. Impact Factor: 0.737.
- Bakhtyukov, A.A.; Derkach, K. V; Fokina, E.A.; Sorokoumov, V.N.; Zakharova, I.O.; Bayunova, L. V; Shpakov, A.O. Development of Low-Molecular-Weight Allosteric Agonist of Thyroid-Stimulating Hormone Receptor with Thyroidogenic Activity. Dokl. Biochem. Biophys. 2022, 503, 67–70, doi: 10.1134/S1607672922020016. Impact Factor: 0.834.
- Bakhtyukov, A.A.; Derkach, K. V; Sorokoumov, V.N.; Stepochkina, A.M.; Romanova, I. V; Morina, I.Y.; Zakharova, I.O.; Bayunova, L. V; Shpakov, A.O. The effects of separate and combined treatment of male rats with type 2 diabetes with metformin and orthosteric and allosteric agonists of luteinizing hormone receptor on steroidogenesis and spermatogenesis. Int. J. Mol. Sci. 2022, 23, doi: 10.3390/ijms23010198. Impact Factor: 6.208.
- Bakhtyukov, A.A.; Derkach, K.V.; Stepochkina, A.M.; Sorokoumov, V.N.; Bayunova, L.V.; Lebedev, I.A.; Shpakov, A.O. Effects of metformin and lutheinizing hormone receptor agonists on steroidogenesis and spermatogenesis in rats with type 2 diabetes with their separate and combined administration. Metabolism 2022, 128, 155010, doi: 10.1016/j.metabol.2021.155010. Impact Factor: 13.934.
- Bakhtyukov, A.A.; Morina, I.Y.; Derkach, K. V.; Romanova, I. V.; Sorokoumov, V.N.; Shpakov, A.O. Development of Approaches to Reducing the Effective Gonadotropin Dose in Treating Androgen Insufficiency in Male Rats with Type 1 Diabetes Mellitus. J. Evol. Biochem. Physiol. 2022, 58, 1503–1513, doi: 10.1134/S0022093022050209. Impact Factor: 1.621.
- Stepochkina, A.M.; Bakhtyukov, A.A.; Derkach, K. V.; Sorokoumov, V.N.; Shpakov, A.O. A Comparative Study of the Steroidogenic Effect of 5-Amino-N-tert-butyl-2-(methylthio)-4-(3-(nicotinamido)phenyl)thieno[2,3-d]-pyrimidine-6-carboxamide and Chorionic Gonadotropin with Different Methods of Administration to Male Rats. J. Evol. Biochem. Physiol. 2022, 58, 54–63, doi: 10.1134/S0022093022010057. Impact Factor: 1.621.
2021
- Mikhaylov, V.N.; Kazakov, I. V.; Parfeniuk, T.N.; Khoroshilova, O. V.; Scheer, M.; Timoshkin, A.Y.; Balova, I.A. The carbene transfer to strong Lewis acids: copper is better than silver. Dalt. Trans. 2021, 50, 2872–2879. doi: 10.1039/D1DT00235J.
- Efremova, M.M.; Govdi, A.I.; Frolova, V.V.; Rumyantsev, A.M.; Balova, I.A. Design and Synthesis of New 5-aryl-4-Arylethynyl-1H-1,2,3-triazoles with Valuable Photophysical and Biological Properties. Molecules. 2021, 26, 2801. doi: 10.3390/molecules26092801.
- V. N. Mikhaylov, I. A. Balova. Alternative Transformations of N-Heterocyclic Carbene Complexes of the Group 11 Metals in Transmetalation Reactions (A Review). Russian Journal of General Chemistry. 2021, Vol. 91, No. 11, pp. 2192–2246. DOI: 10.1134/S1070363221110098.
- Danilkina N.A., Govdi A.I., Khlebnikov A. F., Tikhomirov A.O. Sharoyko V. V., Shtyrov A. A., Ryazantsev M. N. Bräse S., Balova I. A. Heterocycloalkynes Fused to a Heterocyclic Core: Searching for an Island with Optimal Stability-Reactivity Balance. J. Am. Chem. Soc. 2021, 143, 16519–16537. doi: 10.1021/jacs.1c06041.
- Danilkina, N.A.; Andrievskaya, E.V.; Vasileva, A.V.; Lyapunova, A.G.; Rumyantsev, A.M.; Kuzmin, A.A.; Bessonova, E.A.; Balova, I.A. 4-Azidocinnoline—Cinnoline-4-amine Pair as a New Fluorogenic and Fluorochromic Environment-Sensitive Probe. Molecules. 2021, 26, 7460. doi: 10.3390/molecules26247460.
- A.A. Bakhtyukov, K.V. Derkach, I.V. Romanova, V.N. Sorokoumov, T.V. Sokolova, A.I. Govdi, I. Yu. Morina, A.A. Perminova, A.O. Shpakov. Effect of Low-Molecular-Weight Allosteric Agonists of the Luteinizing Hormone Receptor on Its Expression and Distribution in Rat Testes. J Evol Biochem Phys. 2021, 57, 208–220. doi: 10.1134/S0022093021020034.
- Bakhtyukov A.A., Derkach K.V., Stepochkina A.M., Sorokoumov V.N., Bayunova L.V., Lebedev I.A., Shpakov A.O. / The effect of metformin therapy on luteinizing hormone receptor agonists-induced stimulation of testosterone production and spermatogenesis in diabetic rats. J Evol Biochem Phys. 2021, V. 57. № 6. P. 1382–1393. doi: 10.1134/S002209302106017X.
- 12. Derkach, K.V., Romanova, I.V., Bakhtyukov, A.A., Morina, I.Y., Dar’in, D.V., Sorokoumov, V.N., Shpakov, A.O. / The Effect of Low-Molecular-Weight Allosteric Agonist of Luteinizing Hormone Receptor on Functional State of the Testes in Aging and Diabetic Rats. Bulletin of Experimental Biology and Medicine, 2021,171 (1), pp. 81-86. doi: 10.1007/s10517-021-05177-5.
2020
- Mikhaylov, V. N.; Pavlov, A. O.; Ogorodnov, Y. V; Spiridonova, D. V; Sorokoumov, V. N.; Balova, I. A. N-Propargylation and Copper(I)-Catalyzed Azide-Alkyne Cycloaddition as a Convenient Strategy for Directed Post-Synthetic Modification of 4-Oxo-1,4-Dihydrocinnoline Derivatives. Chem. Heterocycl. Compd. 2020, 56 (7), 915–922, doi: 10.1007/s10593-020-02750-0.
- Danilkina, N. A., D’yachenko, A., Govdi, A. I., Khlebnikov, A. F., Kornyakov, I., Bräse, S., & Balova, I. A. (2020). Intramolecular Nicholas Reactions in the Synthesis of Heteroenediynes fused to Indole, Triazole and Isocoumarin. The Journal of Organic Chemistry, doi: 10.1021/acs.joc.0c00930.
- Danilkina, N. A.; Govdi, A. I.; Balova, I. A. 5-Iodo-1H-1,2,3-triazoles as Versatile Building Blocks. Synthesis (Stuttg). 2020, 11–16, doi: 10.1055/s-0039-1690858.
- Danilkina, N. A.; Vasileva, A. A.; Balova, I. A. A.E.Favorskii’s scientific legacy in modern organic chemistry: prototropic acetyleneallene isomerization and the acetylene zipper reaction. Russ. Chem. Rev. 2020, 89, 125–171, doi: 10.1070/rcr4902.
- Mikhaylov, V. N.; Sorokoumov, V. N.; Novikov, A. S.; Melnik, M. V; Tskhovrebov, A. G.; Balova, I. A. Intramolecular hydrogen bonding stabilizes trans-configuration in a mixed carbene/isocyanide PdII complexes. J. Organomet. Chem. 2020, 121174, doi: 10.1016/j.jorganchem.2020.121174.
- Gordeychuk, D. I.; Sorokoumov, V. N.; Mikhaylov, V. N.; Panov, M. S.; Khairullina, E. M.; Melnik, M. V.; Kochemirovsky, V. A.; Balova, I. A. Copper-Based Nanocatalysts Produced via Laser-Induced Ex Situ Generation for Homo- and Cross-Coupling Reactions. Chem. Eng. Sci. 2020, 227, 115940. doi: 10.1016/j.ces.2020.115940.
2019
- Tskhovrebov, A. G.; Novikov, A. S.; Odintsova, O. V.; Mikhaylov, V. N.; Sorokoumov, V. N.; Serebryanskaya, T. V.; Starova, G. L. Supramolecular polymers derived from the PtII and PdII schiff base complexes via C(sp2)–H … Hal hydrogen bonding: Combined experimental and theoretical study. J. Organomet. Chem. 2019, 886, 71–75, doi: 10.1016/j.jorganchem.2019.01.023.
- Danilkina, N. A.; Bukhtiiarova, N. S.; Govdi, A. I.; Vasileva, A. A.; Rumyantsev, A. M.; Volkov, A. A.; Sharaev, N. I.; Povolotskiy, A. V.; Boyarskaya, I. A.; Kornyakov, I. V.; Tokareva, P. V.; Balova, I. A. Synthesis and Properties of 6-Aryl-4-azidocinnolines and 6-Aryl-4-(1,2,3-1H-triazol-1-yl)cinnolines. Molecules 2019, 24, 2386, doi: 10.3390/molecules24132386.
- Danilkina, N.; Rumyantsev, A.; Lyapunova, A.; D’yachenko, A.; Khlebnikov, A.; Balova, I. 10-Membered Azaenediyne Fused to a Benzothiophene through the Nicholas Macrocyclization: Synthesis and DNA Cleavage Ability. Synlett 2019, 30, 161–166. DOI: 10.1055/s-0037-1610352.
- Govdi, A. I.; Danilkina, N. A.; Ponomarev, A. V.; Balova, I. A. 1-Iodobuta-1,3-diynes in Copper-Catalyzed Azide–Alkyne Cycloaddition: A One-Step Route to 4-Ethynyl-5-iodo-1,2,3-triazoles. J. Org. Chem. 2019, 84, 1925–1940. DOI: 10.1021/acs.joc.8b02916.
2018
- Kulyashova, A. E.; Ponomarev, A. V.; Selivanov, S. I.; Khlebnikov, A. F.; Popik, V. V.; Balova, I. A. Cr(II)-promoted internal cyclization of acyclic enediynes fused to benzo[b]thiophene core: Macrocycles versus 2-methylenecycloalkan-1-ols formation. Arab. J. Chem. 2018. DOI: 10.1016/j.arabjc.2018.05.005.
- Mikhaylov, V.; Sorokoumov, V.; Liakhov, D.; Tskhovrebov, A.; Balova, I. Polystyrene-Supported Acyclic Diaminocarbene Palladium Complexes in Sonogashira Cross-Coupling: Stability vs. Catalytic Activity. Catalysts 2018, 8, 141. DOI: 10.3390/catal8040141.
- Tskhovrebov, A. G.; Vasileva, A. A.; Goddard, R.; Riedel, T.; Dyson, P. J.; Mikhaylov, V. N.; Serebryanskaya, T. V.; Sorokoumov, V. N.; Haukka, M. Palladium(II)-Stabilized Pyridine-2-Diazotates: Synthesis, Structural Characterization, and Cytotoxicity Studies. Inorg. Chem. 2018, 57, 930–934. DOI: 10.1021/acs.inorgchem.8b00072.
- Zakharova, I. O.; Sorokoumov, V. N.; Bayunova, L. V; Derkach, K. V; Shpakov, A. O. 4-oxo-1,4-dihydrocinnoline Derivative with Phosphatase 1B Inhibitor Activity Enhances Leptin Signal Transduction in Hypothalamic Neurons. J. Evol. Biochem. Physiol. 2018, 54, 273–280. DOI: 10.1134/S0022093018040038.
- Konovalov, A. I.; Antipin, I. S.; Burilov, V. A.; Madzhidov, T. I.; Kurbangalieva, A. R.; Nemtarev, A. V.; Solovieva, S. E.; Stoikov, I. I.; Mamedov, V. A.; Zakharova, L. Y.; Gavrilova, E. L.; Sinyashin, O. G.; Balova, I. A.; Vasilyev, A. V.; Zenkevich, I. G.; Krasavin, M. Y.; Kuznetsov, M. A.; Molchanov, A. P.; Novikov, M. S.; Nikolaev, V. A.; Rodina, L. L.; Khlebnikov, A. F.; Beletskaya, I. P.; Vatsadze, S. Z.; Gromov, S. P.; Zyk, N. V.; Lebedev, A. T.; Lemenovskii, D. A.; Petrosyan, V. S.; Nenaidenko, V. G.; Negrebetskii, V. V.; Baukov, Y. I.; Shmigol’, T. A.; Korlyukov, A. A.; Tikhomirov, A. S.; Shchekotikhin, A. E.; Traven’, V. F.; Voskresenskii, L. G.; Zubkov, F. I.; Golubchikov, O. A.; Semeikin, A. S.; Berezin, D. B.; Stuzhin, P. A.; Filimonov, V. D.; Krasnokutskaya, E. A.; Fedorov, A. Y.; Nyuchev, A. V.; Orlov, V. Y.; Begunov, R. S.; Rusakov, A. I.; Kolobov, A. V.; Kofanov, E. R.; Fedotova, O. V.; Egorova, A. Y.; Charushin, V. N.; Chupakhin, O. N.; Klimochkin, Y. N.; Osyanin, V. A.; Reznikov, A. N.; Fisyuk, A. S.; Sagitullina, G. P.; Aksenov, A. V.; Aksenov, N. A.; Grachev, M. K.; Maslennikova, V. I.; Koroteev, M. P.; Brel’, A. K.; Lisina, S. V.; Medvedeva, S. M.; Shikhaliev, K. S.; Suboch, G. A.; Tovbis, M. S.; Mironovich, L. M.; Ivanov, S. M.; Kurbatov, S. V.; Kletskii, M. E.; Burov, O. N.; Kobrakov, K. I.; Kuznetsov, D. N. Modern Trends of Organic Chemistry in Russian Universities. Russ. J. Org. Chem. 2018, 54, 157–371. DOI: 10.1134/S107042801802001X.
- Lyapunova, A. G.; Danilkina, N. A.; Rumyantsev, A. M.; Khlebnikov, A. F.; Chislov, M. V.; Starova, G. L.; Sambuk, E. V.; Govdi, A. I.; Bräse, S.; Balova, I. A. Relative Reactivity of Benzothiophene-Fused Enediynes in the Bergman Cyclization. J. Org. Chem. 2018, 83, 2788–2801. DOI: 10.1021/acs.joc.7b03258.
2017
- Shpakova, E. A.; Sorokoumov, V. N.; Akent’ev, A. V.; Derkach, K. V.; Tennikova, T. B.; Shpakov, A. O. The relationship between micelle formation and biological activity of peptide 562–572 of luteinizing hormone receptor modified with decanoyl radicals. Cell tissue biol. 2017, 11, 227–233. DOI: 10.1134/S1990519X17030105.
- Sorokoumov, V. N.; Shpakov, A. O. Protein phosphotyrosine phosphatase 1B: Structure, function, role in the development of metabolic disorders and their correction by the enzyme inhibitors. J. Evol. Biochem. Physiol. 2017, 53, 259–270. DOI: 10.1134/S0022093017040020.
- Gordeychuk, D. Vladimir Kochemirovsky, Victor Sorokoumov, Ilya Tumkin, Alexey Kuzmin, Irina Balova. Copper Particles Generated During in situ Laser-induced Synthesis Exhibit Catalytic Activity Towards Formation of Gas Phase. J. Laser Micro/Nanoengineering 2017, 12, 57–61. DOI: 10.2961/jlmn.2017.02.0001.
- Mikhaylov, V. N.; Sorokoumov, V. N.; Balova, I. A. Polystyrene-supported diaminocarbene complexes of palladium(II): synthesis, characterization and application as a precatalyst in Sonogashira–Hagihara and Suzuki–Miyaura cross coupling. Russ. Chem. Rev. 2017, 86, 459–473. DOI: 10.1070/RCR4715.
- Lyapunova, A. G.; D’yachenko, A. S.; Danilkina, N. A. Potassium fluoride for one-pot desilylation and the Sonogashira coupling of ethynylsilanes and buta-1,3-diynylsilanes. Russ. J. Org. Chem. 2017, 53, 800–804. DOI: 10.1134/S1070428017050268.
- Govdi, A. I.; Kulyashova, A. E.; Vasilevsky, S. F.; Balova, I. A. Functionalized buta-1,3-diynyl-N-methylpyrazoles by sequential “diacetylene zipper” and Sonogashira coupling reactions. Tetrahedron Lett. 2017, 58, 762–765. DOI: 10.1016/j.tetlet.2017.01.032.
2016
- Danilkina, N. A.; Gurskaya, L. Y.; Vasilyev, A. V; Balova, I. A. Towards Isocoumarin-Fused Enediyne Systems through the Electrophilic Cyclization of Methyl o-(Buta-1,3-diynyl)benzoates. European J. Org. Chem. 2016, 739–747. DOI: 10.1002/ejoc.201501262.
- Lyapunova, A. G.; Danilkina, N. A.; Khlebnikov, A. F.; Köberle, B.; Bräse, S.; Balova, I. A. Oxaenediynes through the Nicholas-Type Macrocyclization Approach. European J. Org. Chem. 2016, 2016, 4842–4851. DOI: 10.1002/ejoc.201600767.
- Mikhaylov, V. N.; Sorokoumov, V. N.; Korvinson, K. A.; Novikov, A. S.; Balova, I. A. Synthesis and Simple Immobilization of Palladium(II) Acyclic Diaminocarbene Complexes on Polystyrene Support as Efficient Catalysts for Sonogashira and Suzuki–Miyaura Cross-Coupling. Organometallics 2016, 35, 1684–1697. DOI: 10.1021/acs.organomet.6b00144.
- Mikhailov, V. N.; Korvinson, K.; Sorokoumov, V. N. Chiral acyclic diaminocarbene complexes of palladium(II) immobilized on a polymeric support as promising catalysts of the Suzuki reaction. Russ. J. Gen. Chem. 2016, 86, 2473–2476. DOI: 10.1134/S1070363216110128.
2009–2015
- Danilkina, N. A.; Vlasov, P. S.; Vodianik, S. M.; Kruchinin, A. A.; Vlasov, Y. G.; Balova, I. A. Synthesis and chemosensing properties of cinnoline-containing poly(arylene ethynylene)s. Beilstein J. Org. Chem. 2015, 11, 373–384. DOI: 10.3762/bjoc.11.43.
- Danilkina, N. A.; Lyapunova, A. G.; Khlebnikov, A. F.; Starova, G. L.; Bräse, S.; Balova, I. A. Ring-Closing Metathesis of Co2(CO)6–Alkyne Complexes for the Synthesis of 11-Membered Dienediynes: Overcoming Thermodynamic Barriers. J. Org. Chem. 2015, 80, 5546–5555. DOI: 10.1021/acs.joc.5b00409.
- Kulyashova, A. E.; Mikheeva, E. V.; Danilkina, N. A.; Balova, I. A. Synthesis of 2-(buta-1,3-diynyl)-N,N-dimethylanilines Using Reductive Methylation Step. Mendeleev Commun. 2014, 24, 102–104. DOI: 10.1016/j.mencom.2014.03.013.
- Danilkina, N. A.; Kulyashova, A. E.; Khlebnikov, A. F.; Bräse, S.; Balova, I. A. Electrophilic Cyclization of Aryldiacetylenes in the Synthesis of Functionalized Enediynes Fused to a Heterocyclic Core. J. Org. Chem. 2014, 79, 9018–9045. DOI: 10.1021/jo501396s.
- Mikhailov, V. N.; Savicheva, E. A.; Sorokoumov, V. N.; Boyarskii, V. P. Catalytic activity of palladium(II) diaminocarbene complexes in the Sonogashira and Suzuki reactions. Russ. J. Org. Chem. 2013, 49, 551–554. DOI: 10.1134/S107042801304009X.
- Ryabukhin, D. S.; Sorokoumov, V. N.; Savicheva, E. A.; Boyarskiy, V. P.; Balova, I. A.; Vasilyev, A. V. Catalytic activity of palladium acyclic diaminocarbene complexes in the synthesis of 1,3-diarylpropynones via Sonogashira reaction: cross- versus homo-coupling. Tetrahedron Lett. 2013, 54, 2369–2372. DOI: 10.1016/j.tetlet.2013.02.086.
- Kulyashova, A. E.; Sorokoumov, V. N.; Popik, V. V; Balova, I. A. An acetylene zipper—Sonogashira reaction sequence for the efficient synthesis of conjugated arylalkadiynols. Tetrahedron Lett. 2013, 54, 2235–2238. DOI: 10.1016/j.tetlet.2013.02.066.
- Danilkina, N. A.; Kulyashova, A. E.; Balova, I. A. Intramolecular cyclizations of functionalized diynes. Chem. Heterocycl. Compd. 2012, 48, 95–106. DOI: 10.1007/s10593-012-0973-7.
- Danilkina, N.; Nieger, M.; Selivanov, S.; Bräse, S.; Balova, I. Electrophilic Cyclization and Ring-Closing Metathesis as Key Steps in the Synthesis of a 12-Membered Cyclic Enediyne. European J. Org. Chem. 2012, 2012, 5660–5664. DOI: 10.1002/ejoc.201200881.
- Danilkina, N. A.; Gorbunova, E. G.; Sorokoumov, V. N.; Balova, I. A. Study of cyclyzation of o-(1-Alkynyl)- and o-(1,3-Butadiynyl)aryltriazenes under the action of acids. Russ. J. Org. Chem. 2012, 48, 1424–1434. DOI: 10.1134/S1070428012110048.
- Danilkina, N. A.; Mikhaylov, L. E.; Ivin, B. A. Reaction of acetylenedicarboxylic acids esters with 4,5-dihydro-1H-pyrazole-1-carbothioamides and 3,4,5,6-tetrahydro-2H-1,2,4-triazepine-3-thiones. Chem. Heterocycl. Compd. 2011, 47, 886–900. DOI: 10.1007/s10593-011-0850-9.
- Novikov, R. V.; Danilkina, N. A.; Balova, I. A. Cyclocondensation of n-(prop-2-yn-1-yl)- and n-(penta-2,4-diyn-1-yl)- o-phenylenediamines with phenyl isothiocyanate and carbon disulfide. Chem. Heterocycl. Compd. 2011, 47, 758–766. DOI: 10.1007/s10593-011-0831-z.
- Sorokoumov, V. N.; Popik, V. V.; Balova, I. A. Access to 2,3-bis(buta-1,3-diynyl)pyridines. Mendeleev Commun. 2011, 21, 19–20. DOI: 10.1016/j.mencom.2011.01.008.
- Tskhovrebov, A. G.; Luzyanin, K. V.; Kuznetsov, M. L.; Sorokoumov, V. N.; Balova, I. A.; Haukka, M.; Kukushkin, V. Y. Substituent R-Dependent Regioselectivity Switch in Nucleophilic Addition of N-Phenylbenzamidine to PdII- and PtII-Complexed Isonitrile RN≡C Giving Aminocarbene-Like Species. Organometallics 2011, 30, 863–874. DOI: 10.1021/om101041g.
- Danilkina, N.; Bräse, S.; Balova, I. Electrophilic Cyclization of Buta-1,3-diynylarenes: Synthesis of Precursors of (Z)-3-Ene-1,5-diyne Systems Fused to Heterocycles. Synlett 2011, 2011, 517–520. DOI: 10.1055/s-0030-1259547.
- Vinogradova, O. V.; Sorokoumov, V. N.; Balova, I. A. A short route to 3-alkynyl-4-bromo(chloro)cinnolines by Richter-type cyclization of ortho-(dodeca-1,3-diynyl)aryltriaz-1-enes. Tetrahedron Lett. 2009, 50, 6358–6360. DOI: 10.1016/j.tetlet.2009.08.103.
