 |
Bandura AndreyPhD, Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Head of Department Evarestov RobertDoctor of Physico-Mathematical Sciences, Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
Kuruch DmitryPhD, Assistant
This email address is being protected from spambots. You need JavaScript enabled to view it. |
|
 |
Luk'yanov SergeyPhD, Research Engineer This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Panin AndreyPhD, Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Porsev VitaliyPhD, Associate Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Yakimansky AleksanderDoctor of Chemical Sciences, Professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
First-principles calculations of phonon spectra and thermodynamic properties of transition metal sulfide-based nanotubes
Grant of the Russian Foundation for Basic Research (No. 17-03-00130-a)
Principal investigator: Doctor of Physics and Mathematics, Professor Robert A. Evarestov.
Executors: A. V. Bandura, S. I. Lukyanov, V. V. Porsev, A. V. Kovalenko, D. D. Kuruch
The aim of this project is the development and application of ab initio quantum chemistry methods for calculating thermodynamic properties of nanotubes based on layered transition metal sulfides of 5-th and 6-th periods. Selection of these substances is due to the fact that nanostructures synthesized for transition metal sulfides (TMS) have a great range of properties that can be used in different fields of engineering and technology, such as tribology, catalysis, optoelectronics, and manufacture of batteries, ultracapacitors, and thermoelectric devises. Despite the similarity of their atomic structure, TMS demonstrate a wide range of electronic properties. For example, crystalline zirconium, hafnium, molybdenum and tungsten disulfides are semiconductors; titanium disulfide is semi-metal; whereas niobium and tantalum disulfides are metals. Nanoscale TMS systems have properties that are different from those in the crystalline state. Choosing the composition, morphology, and size of TMS nanosystems, one can strengthen the selected useful properties.
In the first phase of this project we will perform the theoretical analysis of symmetry of phonons and their activity in the IR and Raman spectra of nanotubes based on 3-plane layers with composition MS2 (M = Zr, Hf, Mo, W). Choosing of the metals of 4th and 6th groups is due to the fact that the stable phases of layered sulfides have the different monolayer structure. All considered monolayers are composed of three atomic planes with central plane formed by metal atoms and two outer planes formed by sulfur atoms. However, zirconium and hafnium atoms possess the octahedral coordination, while molybdenum and tungsten atoms have the trigonal prismatic coordination (see fig. 1). Accordingly, we will perform the analysis of the vibrational states of hexagonal layers, resulting from different space groups P-3m (for 1T polymorphic modifications in case of Zr and Hf) and P63/mmc (for 2H polymorphic modification in the case of Mo and W). Consequently, the dynamical representations of corresponding layer groups will be obtained and the symmetry of phonons active IR and Raman spectra of layers will be compared with those for bulk crystals. Diperiodic symmetry group of layers will be used to determine the families of monoperiodic symmetry groups relevant to single-walled nanotubes of different chirality (fig. 2) and their dynamical representations, which will allow us to determine the active IR and Raman spectra of nanotubes in comparison with those for layers. These data are necessary to predict and interpret experimental vibrational spectra of nanotubes considered.
The main goal of our study is to perform the first-principles calculations of phonon spectra of the layers and nanotubes mentioned above. Phonon spectra of layers and nanotubes will be calculated at various points in the Brillouin zone (BZ) to obtain the dispersion curves of phonon states. One of the important objectives of the project is to study the differences between the vibrational spectra of tubes and layers. Quantum chemical calculations would yield stricter quantitative formulation of these differences for the specific systems.
The phonon frequencies obtained by ab initio calculations is to be used for assessing the thermodynamic properties of layers and nanotubes (by summing the relevant contributions over set of BZ points) at different temperatures. These computations allow the direct comparison of thermodynamic properties (Helmholtz energy, entropy and heat capacity) of flat layers and nanotubes obtained by their folding. Knowledge of free energies will enable the correct estimate of the nanotube relative stability and study its temperature dependence. The calculation of thermal expansion coefficients, as well as modules of the elasticity of layers and nanotubes is also planned.
Relevant publications
- A. V. Bandura, V. V. Porsev, R. A. Evarestov, Application of Zone-Folding Approach to the First-Principles Estimation of Thermodynamic Properties of Carbon and ZrS2-Based Nanotubes. J. Comput. Chem. 2016, 37, 641–652. DOI: http://dx.doi.org/10.1002/jcc.24243.
- A. V. Bandura, D. D. Kuruch, R. A. Evarestov, First-principles Calculations of InS-based Nanotubes. Isr. J. Chem. 2017, 57, 490– 500. DOI: http://dx.doi.org/10.1002/ijch.201600054
- R. A. Evarestov, A. V. Bandura, V. V. Porsev, A. V. Kovalenko, Phonon Spectra, Electronic, and Thermodynamic Properties of WS2 Nanotubes. J. Comput. Chem. 2017, in print. DOI: http://dx.doi.org/10.1002/jcc.24916
Figure Captions
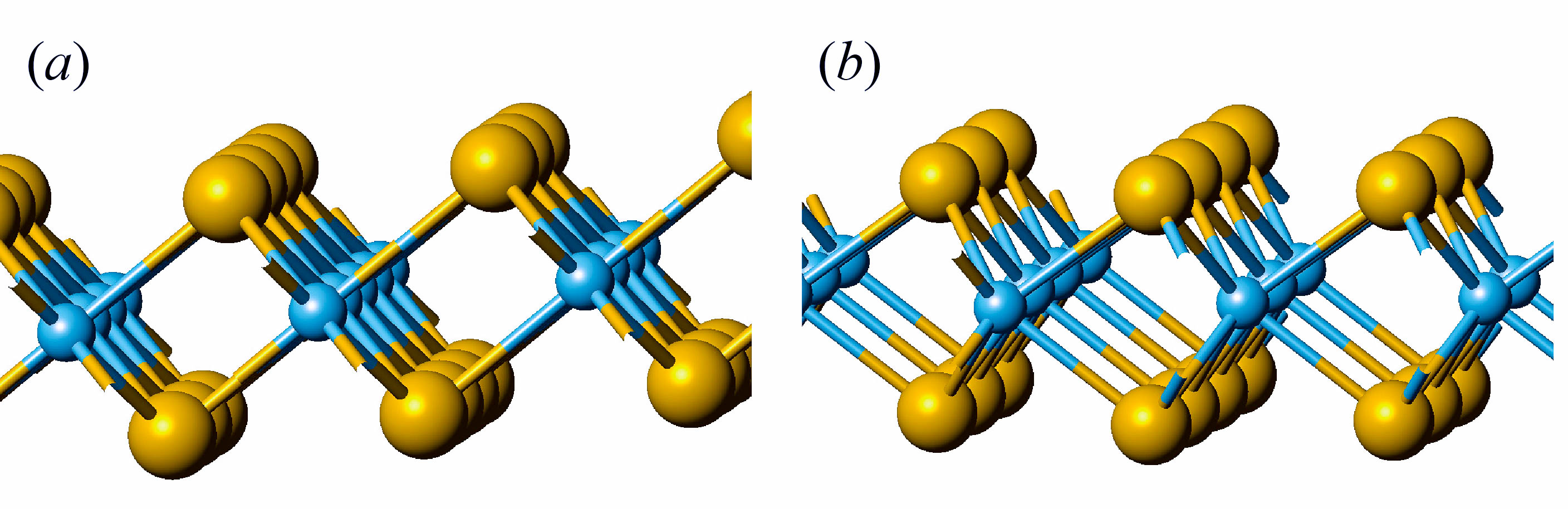
Fig. 1. Monolayer structure of ZrS2 with hexagonal Zr coordination (a), and MoS2 with trigonal-prismatic coordination of Mo (b). Sulfur atoms – large spheres, metal atoms – small spheres.
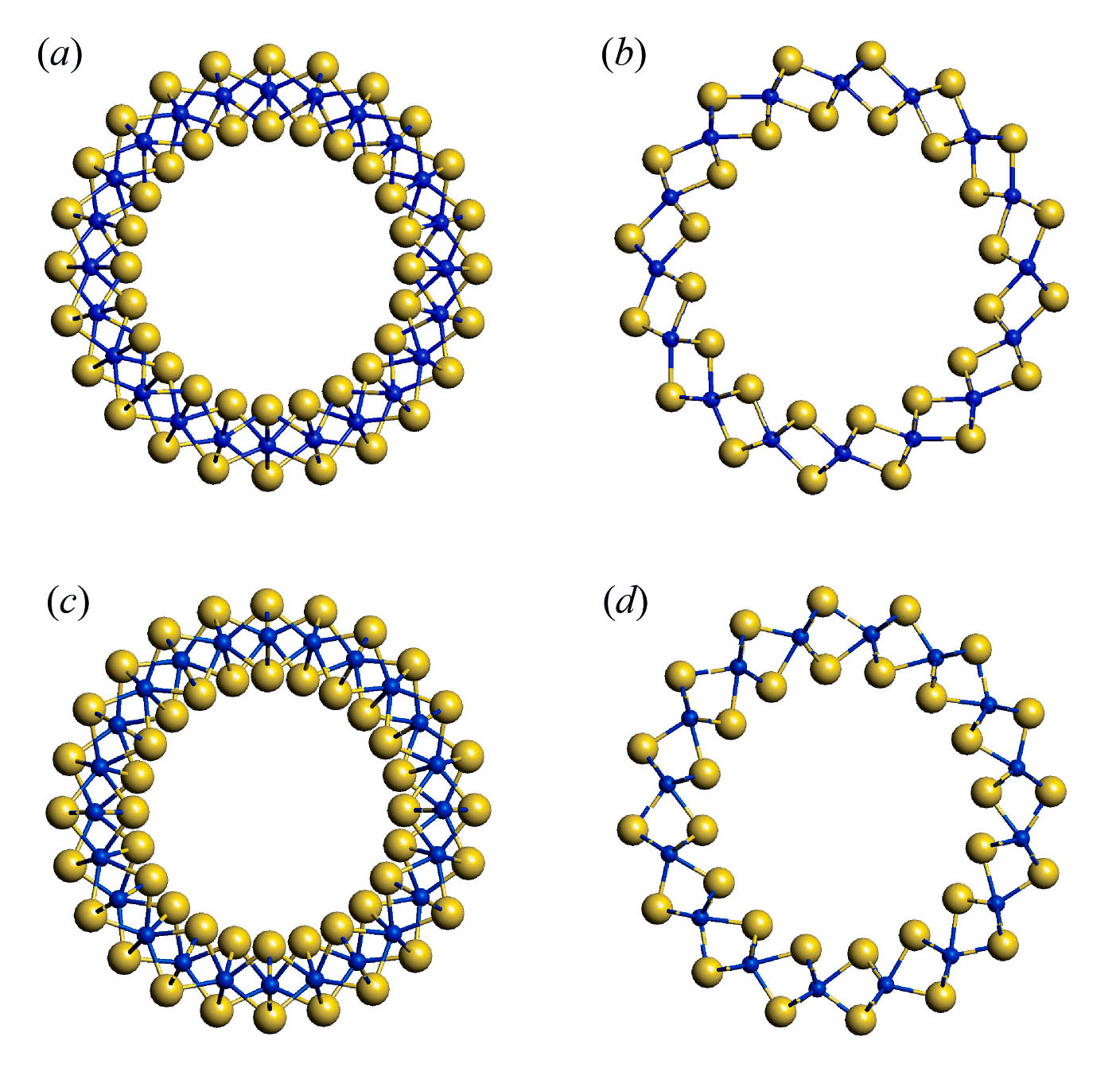
Fig. 2. Cross-section view of nanotubes obtained by rolling up of monolayers of ZrS2 (a, b) and MoS2 (c, d) with chirality (12, 0) (a, c) and (8, 8) (b, d). Sulfur atoms – large spheres, metal atoms – small spheres.
- A. V. Bandura, S. I. Lukyanov, R. A. Evarestov, Atom–atom force field for simulation of zirconia bulk, nanosheets and nanotubes. Mol. Sim. 2017, 43, 886–899. DOI: http://dx.doi.org/10.1080/08927022.2017.1303685
- R. A. Evarestov, A. V. Bandura, V. V. Porsev, A. V. Kovalenko, First-principles modeling of hafnia-based nanotubes. J. Comput. Chem. 2017, 38, 2088–2099. DOI: http://dx.doi.org/10.1002/jcc.24849
- A. V. Bandura, R. A. Evarestov, S. I. Lukyanov, S. Piskunov, Y. F. Zhukovskii, Simulation of Young’s moduli for hexagonal ZnO [0 0 0 1]-oriented nanowires: first principles and molecular mechanical calculations. Mater. Res. Express 2017, 4, 085014. DOI: https://doi.org/10.1088/2053-1591/aa7732
- V. V. Porsev, A. V. Bandura, R. A. Evarestov, Water adsorption on α-V2O5 surface and absorption in V2O5∙nH2O xerogel: DFT study of electronic structure. Surf. Sci. 2017, in print. DOI: https://doi.org/10.1016/j.susc.2017.08.022
- N.Y. Shilyagina, N.N. Peskova, S.A. Lermontova, A.A. Brilkina, V.A. Vodeneev, A.V. Yakimansky, L.G. Klapshina, I.V. Balalaeva. Effective delivery of porphyrazine photosensitizers to cancer cells by polymer brush nanocontainers. J. Biophotonics. 2017. V. 10. No. 9. P. 1189-1197.
- G.I. Nosova, D.M. Ilgach, I.A. Berezin, E.V. Zhukova, T.N. Kopylova, E.N. Nikonova, R.M. Gadirov, R.Yu. Smyslov, A.V. Yakimansky. White electroluminescence from polyfluorenes copolymerized with carbazole derivatives of Nile Red and 1,8-naphthalimide. Mendeleev Commun. 2017. V. 27. No. 4. P. 265-267.
- D.M. Ilgach, G.I. Nosova, T.N. Kopylova, E.N. Nikonova, R.M. Gadirov, R.Yu. Smyslov, L.S. Litvinova, A.V. Yakimansky. Polyfluorene copolymers containing 2,5-difluoro-1,4-phenylene chains and carbazole conjugates with 1,8-naphthalimides for stable blue OLEDs. Mendeleev Commun. 2017. V. 27. No. 4. P. 357-359.
- S.I.Lopatin, S.M.Shugurov A.V.Fedorova, A.V.Utina, A.I.Panin, Mass spectrometric study of thermodynamic properties of BaO-CeO2. The formation enthalpy of BaCeO3 (solid), Journal of Alloys and Compounds, 693 (2017), 1028-1034.
- S. I. Lopatin, S. M. Shugurov, A. I. Panin, E. A. Vasil'eva, Evaluation of relative electron ionization cross-sections for some oxides and oxyacid salts, Rapid Communications in Mass Spectrometry, (2017), 31, 19,1559-1564.
- Evarestov R.A. Book Title THEORETICAL MODELING OF INORGANIC NANOSTRUCTURES: SYMMETRY AND AB-INITIO CALCULATIONS OF NANOLAYERS, NANOTUBES AND NANOWIRES Series Title: Nanoscience and Technology. Number of Pages: 1-672 Published: 2015. Publisher: SPRINGER-VERLAG BERLIN, HEIDELBERGER PLATZ 3, D-14197 BERLIN, GERMANY
- Series Title Springer Series in Solid-State Sciences; Series Volume 153. Book Title Quantum Chemistry of Solids Book Subtitle LCAO Treatment of Crystals and Nanostructures Author: R. A. Evarestov Number of Pages: 1-734 Published: 2012 Publisher: SPRINGER-VERLAG BERLIN, HEIDELBERGER PLATZ 3, D-14197 BERLIN, GERMANY
- Polynomial approximations of electronic wave functions, A. I. Panin, Journal of Mathematical Chemistry, 2011, Volume 49, Issue 8, pp 1599–1623
- Rod groups and their settings as special geometric realisations of line groups. R.A.Evarestov, A.I.Panin Acta Crystallographica Section A: Foundations of Crystallography, 2012. Vol. A68, P. 582-588
- A.I. Gorkovenko, A.I. Plekhanov, A.E. Simanchuk, A.V. Yakimanskiy, G.I. Nosova, N.A. Solovskaya, N.N. Smirnov. Temperature dependence and the dispersion of nonlinear optical properties of chromophore-containing polyimide thin films. J. Appl. Phys. 2014. V. 116. Article Number 223104.
- Quantum Chemical Study of Water Adsorption on the Surfaces of SrTiO3 Nanotubes, Author: Bandura, Andrei V.; Kuruch, Dmitry D.; Evarestov, Robert A. CHEMPHYSCHEM Vol.: 16 Issue: 10. P.: 2192-2198, 2015.
- Young's modulus and Poisson's ratio for TiO2-based nanotubes and nanowires: modelling of temperature dependence. Author: Lukyanov, Sergey I.; Bandura, Andrei V.; Evarestov, Robert A. RSC ADVANCES Vol.: 6 Issue: 19. P.: 16037-16045, 2016.
- F. Bellucci, S. S. Lee, J. D. Kubicki, A. Bandura, Zh. Zhang, D. J. Wesolowski, P. Fenter, Rb+ Adsorption at the Quartz(101)−Aqueous Interface: Comparison of Resonant Anomalous X-ray Reflectivity with ab Initio Calculations. JOURNAL OF PHYSICAL CHEMISTRY C 2015, 119 (9), 4778–4788; DOI: 10.1021/jp510139t.
- N.P. Yevlampieva, A.P. Khurchak, G.I. Nosova, R.Yu. Smyslov, I.A. Berezin, D.M. Ilgach, T.N. Kopylova, R.M. Gadirov, A.V. Yakimansky. Chain microstructure and specific features of excitation energy transfer in solution and films of poly(9,9-dioctylfluorene) dopedwith 2,1,3-benzothiadiazole comonomer units. Chemical Physics Letters. 2016. V. 645. P. 100–105.
- Shugurov S.M., Panin A.I., Lopatin S.I., Emelyanova K.A. Formation and themodynamics of gaseous germanium and tin vanadates: a mass spectrometric and quantum chemical study, Dalton Transactions (2015), 44, 10014-10021.
- Ab initio modeling of single wall nanotubes folded from alpha- and gamma-V2O5 monolayers: structural, electronic and vibrational properties Author: Porsev, V. V.; Bandura, A. V.; Evarestov, R. A. CRYSTENGCOMM V: 17 Issue: 17 P.: 3277-3285, 2015
History of the Quantum Chemistry Department
It became apparent to the end of 60th of the 20th century that there was strong tendency of penetration of physico-mathematical methods to almost all fields of chemistry. In response to this state of affairs the Scientific Council of the University Chemical faculty has decided to establish the first in our country Division of Quantum Chemistry.
The practical steps to realize this decision were undertaken by the Dean of the Chemical Department A. N. Murin and the Director of the Scientific Institute of Chemistry A. G. Morachevskiy with the help of the Physical Department professor, major specialist in quantum mechanics M. G. Veselov. The result of these efforts was the establishment of the Quantum Chemistry Division in 1967-1968. The Physical Department associate professor A. V. Tulub was invited to be a Head of the Division.
The original staff of the Division consists of chemist V. I. Baranovskiy, mathematicion V. F. Bratsev and theorists in physics N. P. Borisova and R. A. Evarestov. This membership of the Division was able to embrace practically all trends of modern Quantum Chemistry.
The first Head of the Division was A. V. Tulub, Doctor of Physics and Mathematics, Professor, Active member of the Russian Academy of Natural Sciences. He is author of more than 120 scientific publications on the various subjects of theoretical physics and chemistry. The sphere of the research interests of A. V. Tulub includes ab initio and semi-empirical calculations of molecular systems polarizability, theory of van der Waals interactions of molecular networks with a surface, calculations of the vibrational spectra of molecules, ab initio calculations of the metastable particles properties, application of quantum electrodynamics methods in calculations of electron density at the nucleus of the atom of heavy elements.
In the present time professor Robert A. Evarestov, Doctor of Physics and Mathematics, honored worker of science of Russia, active member of the Russian Academy of Natural Sciences, foreign member of the Latvian Academy of Sciences Humboldt professor, is the head of Quantum Chemistry Division. He is widely known and esteemed specialist in quantum chemistry, author of 200 scientific publications and six monographs. R. A. Evarestov carries out research in the area of the multi-electron systems theory which have been initiated by the worldwide known Petersburg scientific school of V. A. Fock and his assistants – professors M. I. Petrashen and M. G. Veselov.
He developed a new research direction of the modern theoretical chemistry – quantum chemistry of solid. This direction is based on the application of the molecular models of the crystals and the computational methods of the theory of molecules intended to solve the problems of solid state chemistry.
From the beginning of his work at Quantum Chemistry Division doctor of chemistry, professor, RANS corresponding member Victor Ivanovich Baranovskii investigated the electronic structure of the transition metal complexes. Together with Dr. O. V. Sizova he found out electronic mechanism of trans-influence. These conclusions were based on the results of quantum chemical computations and the perturbation theory application.
The packages of computer programs for semi-empirical and ab initio calculations of the molecular systems properties were created under his leadership. These computer programs were unique instruments in our country at that time. Also the research works on the simple chemical reactions were initiated under his leadership.
Ph.D. in chemistry, associate professor Andrei Ivanovich Panin is one of the first graduates of the Quantum Chemistry Division (1970). The area of his research interests includes electron configuration interaction, calculations of potential energy surfaces of molecular systems, theoretical treatment of gas-phase reactions, mathematical methods of quantum chemistry. He proposed a version of the configurations interaction method including arbitrary restrictions on the occupancies of groups of MOs. The corresponding algorithms and programs were worked out. He suggested new models of the Fock space and demonstrated that the Fock space can be endowed with new multiplications essentially different from Grassmann’s and Clifford’s ones. These new multiplications turned out to be closely related to the non-linear methods of quantum chemistry and, in particular, to the CC method. Using methods of modern differential geometry, A. Panin performed a study of evolution of many electron systems on manifolds in the space of their states and demonstrated how the curvature of the surface where a system evolves is connected with the TD methods of quantum chemistry. At present A. Panin works on the problems connected to the symmetry of 1-periodic systems.
Ph.D. in chemistry, associate professor Sergey Georgievich Semenov is a graduate of the Quantum Chemistry Division, 1971. In 1973-1980 he proposed a theoretical definition of the valency. According to this definition the valency is equal to the doubled dispersion of a number of electrons belonging to an atom of a chemical compound. In 1977 he also proposed a concept of unshared electronic occupation. He worked out generalized method of self-consistent field which makes it possible to take into account the known equalities including the density matrix.
A. V. Bandura and V. V, Porsev, associate professors, also are graduates of the Quantum Chemistry Division. They are members of the research group of prof. R. A. Evarestov and works on the problems of quantum chemistry of the nanostructures: electronic, vibrational and thermodynamic properties of nanolayers, nanotubes, nanowires and nanoribbons.
