Department of Physical Organic Chemistry
Non-covalent Interactions Laboratory
Short URL for media go.spbu.ru/rgtolstoyp
Group members
 |
Dr. Peter M. TolstoyGroup HeadProfessor http://www.researcherid.com/rid/J-2966-2013 This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Dr. Elena Yu. TupikinaAssociate Professor https://orcid.org/0000-0002-0998-8348 This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Dr. Valeriia V. Mulloyarova Teaching Assistant http://orcid.org/0000-0002-5688-2431 rooms 2135, 2130 |
|
Dr. DariaI. Tonkoglazova Researcher https://orcid.org/0000-0002-1643-3471 This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2142 |
|
|
Dr.Tsybulin V. Semyon Researcher https://orcid.org/0000-0001-5033-8454 This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2141 |
|
 |
Mikhail A. Kostin Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2130 |
 |
Edem R. Chakalov PhD student Research engineer This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2130 |
 |
Valerii V. Verkhov PhD student Research engineer rooms 2135, 2126, 2142 |
 |
Omar Alkhuder PhD student rooms 2135, 2130 |
 |
Danil V. Krutin PhD student Research engineer This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2126 |
 |
Anna A. Titova Masterstudent Research engineer room 2126 |
 |
Daniil A. Shitov Masterstudent room 2126 |
 |
Mark V. Kaplanskiy Masterstudent Research engineer room 2126 |
|
Ksenia I. Morozova Masterstudent This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2130 |
|
|
Dmitriy S. Lobanov Masterstudent This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2142 |
|
 |
Stepan A. Meshalkin Bachelorstudent Research engineer This email address is being protected from spambots. You need JavaScript enabled to view it. room 2135, 2126, 2141 |
|
Alexandra N. Gubanova Bachelorstudent Research engineer This email address is being protected from spambots. You need JavaScript enabled to view it. room 2135, 2142 |
|
|
AntonS. Zaharov Bachelorstudent This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2130 |
|
|
MaximL. Kruglov Bachelorstudent This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2142 |
|
|
DenisA. Kamynin Bachelorstudent This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2141 |
|
|
Stepan A. Galiakhmetov Bachelorstudent This email address is being protected from spambots. You need JavaScript enabled to view it. rooms 2135, 2142 |
|
|
Anton P. Godun Bachelorstudent This email address is being protected from spambots. You need JavaScript enabled to view it. room 2135 |
|
|
Former members |
|
 |
Victor G. BardakovGraduate of PhD program |
 |
Alexandra M. PuzykMaster of Chemistry |
 |
Valerii V. KarpovMaster of Chemistry |
 |
Vladislav O. KorostelevMaster of Chemistry |
 |
Denis V. SolovevBachelor student |
 |
Ilja A. TatarinovBachelor student
|
 |
Anna A. TitovaBachelor student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Dmitrii O. TolochenkoMaster of Chemistry |
 |
Artyom A. YakubenkoBachelor of Chemistry |
 |
Dr. Alexander S. AntonovUniversity of Regensburg Researcher https://orcid.org/0000-0001-7047-789X |
 |
Dr.VladimirY. Mikshiev https://orcid.org/0000-0003-1204-0352 |
 |
Ivan S. GibaPhD in physics
|
 |
Alexandra A. EfimovaMaster of physics |
 |
Mikhail A. ZolenkoBachelor student |
 |
Andrey A. SudarkinBachelor of Chemistry |
 |
Daria O. UstimchukBachelor of Chemistry |
 |
Ivan S. Chunarev |
 |
Alexei S. Ostras'Bachelor of chemistry |
 |
Anastasia V. NamMaster of Chemistry
|
Collaborations
| Colleague | Joint Publications |
|
Dr. Alexander Antonov Department of Chemistry, University of Regensburg, Regensburg, Germany
|
A.S. Zakharov, D.V. Krutin, P.O. Mosalyov, E.Yu. Tupikina, A.S. Antonov, P.M. Tolstoy, V.V. Mulloyarova, “Phosphine Selenides: Versatile NMR Probes for Analyzing Hydrogen OH···Se and Halogen I···Se Bonds”, Phys.Chem.Chem.Phys.2024, published online. DOI: 10.1039/D4CP01895H. J. Eder, A.S. Antonov, E.Yu. Tupikina, R.M. Gschwind, “Chiral Diselenophosphoric Acids for Ion Pair Catalysis: A Novel Approach to Enhance Both Proton Donating and Proton Accepting Properties”, Chem. Eur. J. 2024, e202401793. DOI: 10.1002/chem.20240179. S.A. Meshalkin, S.V. Tsybulin, V.G. Bardakov, I.A. Tatarinov, D.A. Shitov, E.Yu. Tupikina, M.M. Efremova, A.S. Antonov, “Buttressing Effect" in the Halogen-Lithium Exchange in ortho-bromo-N,N-dimethylanilines and Related Naphthalenes”, Chem. Eur. J. 2023, e202303956. DOI: 10.1002/chem.202303956. M.V. Kaplanskiy, V.V. Karpov, E.Yu. Tupikina, A.S. Antonov, “NMR Descriptors of the Strained Metallacycles Formation in Organo-lithiums: Theoretical Study”, Organic and Biomolecular Chemistry2023. DOI: 10.1039/d3ob01916k. A. A. Yakubenko, E. Yu. Tupikina, A. S. Antonov, “The Role of Conjugation in the Halogen‐Lithium Exchange Selectivity: lithiation of 4, 6, 7, 9‐tetrabromo‐1, 3‐dimethyl‐2, 3‐dihydro‐1H‐perimidine”, Chem. Eur. J. 2023, 29, e202301439. DOI: 10.1002/chem.202301439.
|
|
Prof. Dr. Vadim Yu. Kukushkin RAS Academician Saint Petersburg State University Institute of Chemistry Department of Physical and Organic Chemistry |
A.VRozhkov, E.Yu. Tupikina, K.I. Tugashov, V.Yu. Kukushkin, « Pure Heterometallic Spodium Bonding», CrystEngComm2024. DOI: 10.1039/D4CE00825A. M.V. Kashina, M. A. Kinzhalov, E.Yu. Tupikina, V.Yu. Kukushkin, “Linear and Bent Noncovalent M··· S═ C═ N–M′ Interactions: The Case of Palladium (II) and Platinum (II) Thiocyanate Species”, Cryst. Growth Des.2023, 23, 4322–4335. DOI: 10.1021/acs.cgd.3c00116. I.S. Aliyarova, E.Yu. Tupikina, N.S. Soldatova, D.M. Ivanov, P.S. Postnikov, M. Yusubov, V. Yu. Kukushkin, «Halogen Bonding Involving Gold Nucleophiles in Different Oxidation States», Inorg. Chem.2022, 61, 39, 15398–15407; DOI: 10.1021/acs.inorgchem.2c01858. |
|
Prof. Dr. Alexander Pozharskii, Department of Organic Chemistry, Southern federal University |
A.V. Marchenko, V.A. Ozeryanskii, O.P. Demidov, A.A. Antonov, E.Yu. Tupikina, A.F. Pozharskii, “Organometallic Synthesis of 2,3,6,7-Tetrasubstituted 1,8-Bis(dimethylamino)naphthalenes for Investigation of the Double Buttressing Effect in Proton Sponges”, J. Org. Chem.2022, 87(24):16506-16516. DOI: 10.1021/acs.joc.2c02212 |
|
Prof. Dr. Mark Sigalov Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel |
E.Yu. Tupikina, M.V. Sigalov, O. Alkhuder, P.M. Tolstoy, “Charge Relay Without Proton Transfer: Coupling of Two Short Hydrogen Bonds via Imidazole in Models of Catalytic Triad of Serine Protease Active Site”, ChemPhysChem2024. DOI: 10.1002/cphc.202300970 E.Yu. Tupikina, M.V. Sigalov, P.M. Tolstoy, «Simultaneous Estimation of Two Coupled Hydrogen Bond Geometries from Pairs of Entangled NMR Parameters: The Test Case of 4-Hydroxypyridine Anion», Molecules2022, 27, 3923. DOI: 10.3390/molecules27123923. |
|
Prof. Dr. Kostas Sotiriadis Institute of Theoretical and Applied Mechanics of the Czech Academy of Sciences, Prague, Czech Republic |
K. Sotiriadis, A.S. Mazur, Peter M. Tolstoy, P. Mácová, A. Viani, “External magnesium sulfate attack on hydrated Portland-limestone cements: Effect of the concurrent presence of sodium chloride in the corrosive environment and metakaolin admixture in the binder”, Cem. Concr. Compos.2024, 151, 105614. DOI: 10.1016/j.cemconcomp.2024.105614. A.Mazur, P. Tolstoy, K. Sotiriadis, “13С, 27Al and 29Si NMR investigation of the hydration kinetics of Portland-limestone cement pastes containing CH3-COO–R+ (R=H or Na)”, Materials 2022, 15, 2004. DOI: 10.3390/ma15062004. K. Sotiriadis, K. Aspiotis, A. Mazur, P. Tolstoy, E. Badogiannis, S. Tsivilis, “Characterization of Old Concrete from a Heritage Structure of Inousses Cluster of Islands”, Lect. Notes Civ. Eng.2022, 209, 80-89. DOI: 10.1007/978-3-030-90788-4_7. |
|
Prof. Dr. Viktor Rozentsvet Institute of Ecology of the Volga River Basin, Russian Academy of Sciences, Tol´yatti, Russia |
V.A. Rozentsvet, N.A. Sablina,D.M. Ulyanova, P.M. Tolstoy, “Structural characterization of lowmolecular weight polybutadiene synthesized using cationic initiationsystem”, Russ. Chem. Bull. 2024, 73(4), 1035-1045. DOI: 10.1007/s11172-024-4218-6. V.A. Rozentsvet, D.M. Ulyanova, N.A. Sablina, S.V. Kostjuk, P.M. Tolstoy, I.A. Novakov, “Cationic polymerization of butadiene using alkyl aluminum compounds as co-initiators: an efficient approach toward solid polybutadienes”, Polym. Chem.2022, 3, 1596-1607. DOI: 10.1039/d1py01684a. V. Rozentsvet, N. Sablina, D. Ulyanova, S. Kostjuk, P. Tolstoy, “Structure of terminal units of polybutadiene synthesized via anionic mechanism”, Polym. Bull.2022, 79, 1239-1256. DOI: 10.1007/s00289-021-03549-5. |
|
Prof. Dr. Alexander Artem’ev Nikolaev Institute of Inorganic Chemistry, Siberian Branch of Russian Academy of Sciences, Novosibirsk, Russia |
E.Yu. Tupikina, M.P. Davydova, V.V. Mulloyarova, T.S. Sukhikh, D.G. Samsonenko, P.M. Tolstoy, A.V. Artem’ev, “Remarkably short intermolecular Se···Se contacts in Ni(II)diselenophosphinates: interplay of electrostatic and dispersion”, Inorg. Chem. Front.2024, under review |
|
Prof. Dr. Elena V. Grachova Saint Petersburg State University Institute of Chemistry Department of General and Inorganic Chemistry |
A. Paderina, S. Slavova, E. Tupikina, D. Snetkov, E. Grachova, «Aggregation game: changing solid-state emission using different counterions in mono-alkynylphosphoniumPt(II) complexes », Inorg. Chem.2024. DOI: 10.1021/acs.inorgchem.4c02130. |
Research
Non-covalent Interactions
Non-covalent interaction is the common name for interactions the formation of which does not result in the commonization of the electron density. The energy of non-covalent interactions is much lower than the energy of covalent ones. However, due to their prevalence, non-covalent interactions have a significant influence on the physical, chemical, and structural properties of molecules. These mobile and variable interactions while often not determining the constancy of the composition of a chemical compound in different phase states, govern many of its physical, chemical, and structural properties. These interactions can be found in biopolymers, liquid crystals, supramolecular aggregates, solutions, liquids, and molecular crystals. Non-covalent interactions are ubiquitous, numerous, and functional: every chemical reaction starts with them and every condensed state of matter is maintained by them.
All known to date non-covalent interactions can be classified into four groups: electrostatic, Van-der-Waals, hydrophobic, and π-interaction.
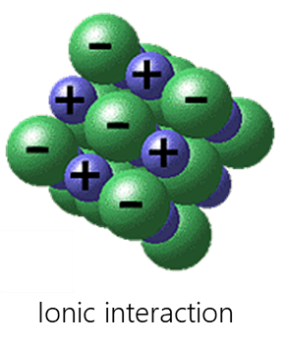
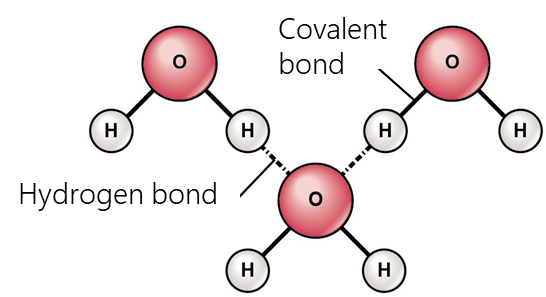
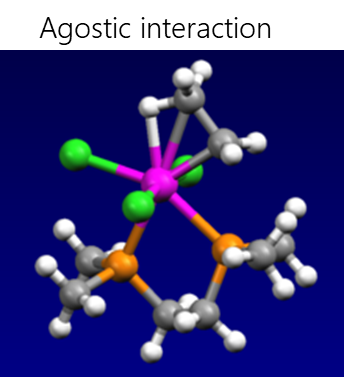
Electrostatic interactions include hydrogen bonding, σ-hole interactions (halogen, pnictogen, halcogen, aerogen, tetrel etc.), agnostic interactions and ionic interactions.
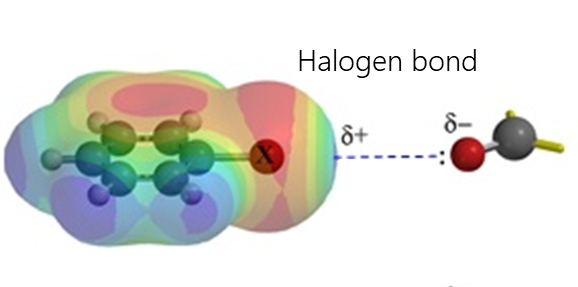
- Halogen bonding occurs due to an attractive interaction between the electrophilic region of the halogen atom (the so-called σ-hole) and the nucleophilic region (such as an unshared electron pair) in another molecule. This interaction is widely used in such fields as supramolecular chemistry, crystallography, and liquid crystal fabrication.
- Hydrogen bonding occurs when a hydrogen atom bonded to a strongly negative atom (fluorine, oxygen, nitrogen, etc.) comes to proximity with another electronegative atom. These bonds are usually stronger than normal dipole-dipole and dispersion forces, but weaker than true covalent and ionic bonds. Hydrogen bonding largely determines the properties of such biologically important substances as proteins and nucleic acids. In particular, secondary structure (e.g., α-helices, β-folds) and tertiary structure in proteins, RNA, and DNA molecules are stabilized by hydrogen bonds.
- Ionic interaction is realized by electrostatic attraction between anions and cations. The most important features of ionic bonding from other types of chemical bonding are its non-directional and non-saturated nature. That is why crystals formed by ionic bonding tend to have various dense packings of the corresponding ions.
- Agostic interaction is the interaction of a coordinationally unsaturated transition metal with the C-H bond, when the two electrons involved in the C-H bond move to the empty d-orbital of the transition metal, resulting in the formation of a three-center two-electron bond. Many catalytic transformations, such as oxidative addition and reductive elimination, presumably involve agostic interactions.
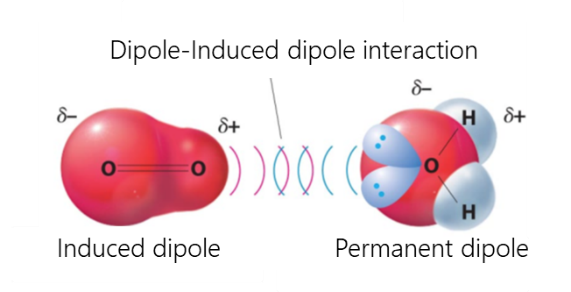
Van-der-Waals interactions include dipole-dipole, dipole-induced dipole, and dispersive interactions.
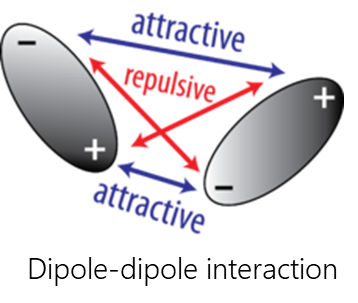
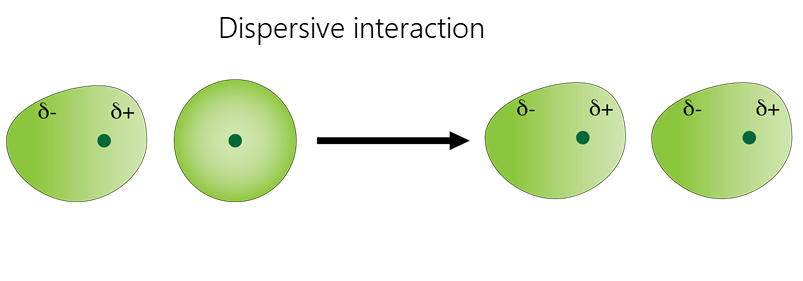
- Dispersive interaction is caused by the interaction of atoms and molecules with each other due to their own or mutually induced dipole moments. The instantaneous charge distribution of one atom or molecule, characterized by an instantaneous dipole moment, induces an instantaneous dipole moment in another atom or molecule
- The interaction between a dipole and an induced dipole occurs when a polar molecule induces a dipole in an atom or in a non-polar molecule, disturbing the arrangement of electrons in the non-polar member.
- Dipole-dipole interactions occur when two polar molecules interact with each other through space. In this case, the partially negative part of one of the polar molecules is attracted to the partially positive part of the second polar molecule.
Hydrophobic interactions are the attraction between non-polar particles in water (or other polar solvents), which is caused by the thermodynamic disadvantage of water contact with non-polar substances. Hydrophobic interaction takes part in the formation of the tertiary structure of proteins and provides molecular recognition in some supramolecular guest-host complexes. It manifests itself in the formation of micelles and other structures in surfactant solutions.

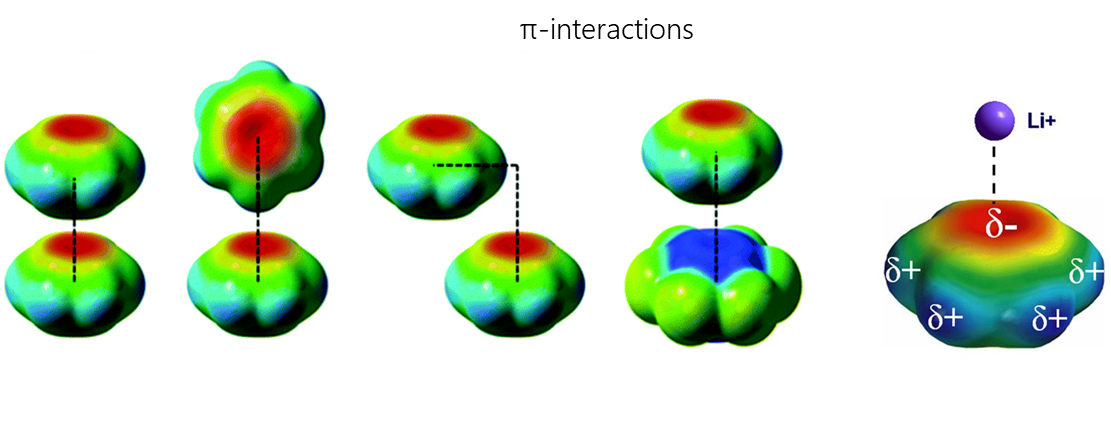
π-interactions are a type of non-covalent interactions in which an electron-rich π-system can interact with a cation, anion or other π-system. Non-covalent interactions with π-systems are crucial for biological processes such as protein-ligand recognition.
Projects
Organometallic Chemistry
Since the discovery of the basic principles of organometallic compounds by Edward Frankland in the second half of the 19th century, the application of these reagents in organic synthesis began to develop rapidly. Today, the organometallic strategy of functionalization makes it possible to carry out complex transformations with high regio- and stereoselectivity and opens the possibility of introducing substituents in positions that are inaccessible by methods of classical chemistry. In order to further expand the synthetic potential of organoelement compounds, the following projects are being implemented in our laboratory:
- Direct lithiation of aromatic amines and related compounds for the synthesis of their hard-to-reach meta- and peri-derivatives.
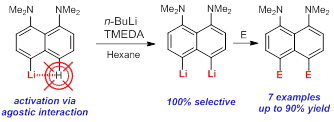 |
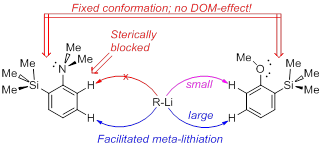 |
| Antonov, Yakubenko Synthesis 2020 | Antonov et al. J. Organomet. Chem. 2020 |
- Study of the structure of organometallic compounds.
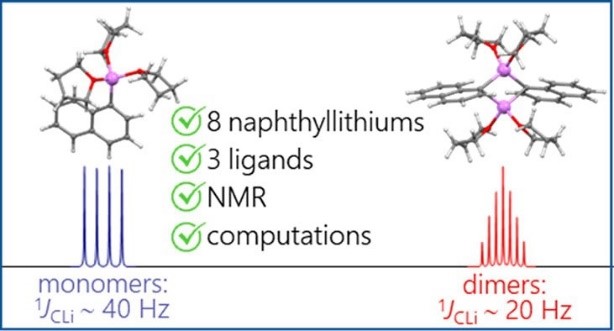
Antonov et al. Organometallics. 2020
- Steric activation of the dimethylamino group in the synthesis of polynuclear nitrogen heterocycles.
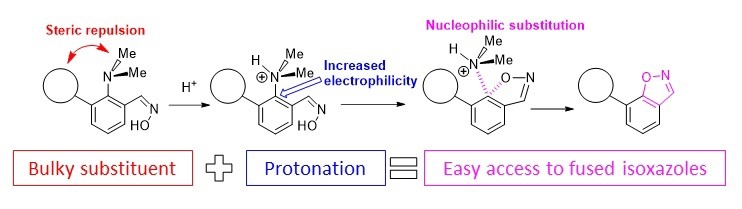
Antonov et al. Molecules 2020
Modern approach to the study of non-covalent interactions requires the development of methods for the synthesis of new donors and acceptors, which will allow to understand the periodicity of changes in the interaction force from the nature of the elements that make up the binding participants, as well as allow the use of new more sensitive methods of investigation. Within the framework of this direction our laboratory implements the following projects:
- Development of methods for the synthesis of organic acids, oxides, and selenides of group 15 elements.
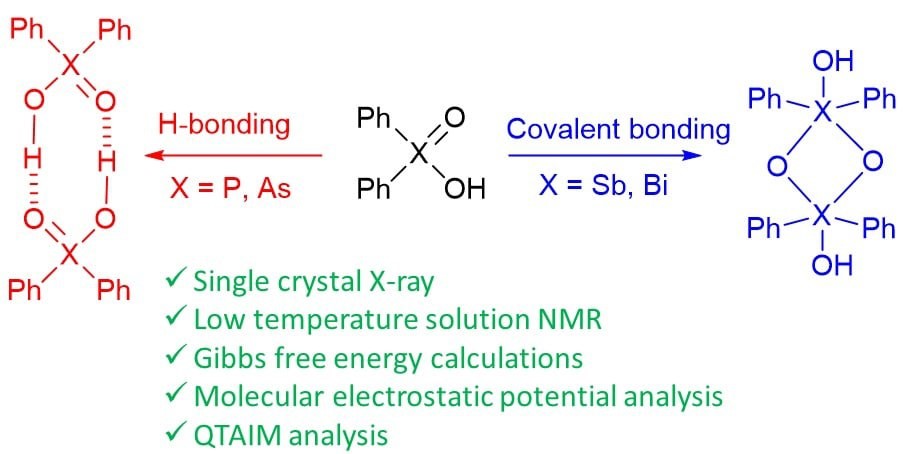
Yakubenko et al. Phys. Chem. Chem. Phys. 2022
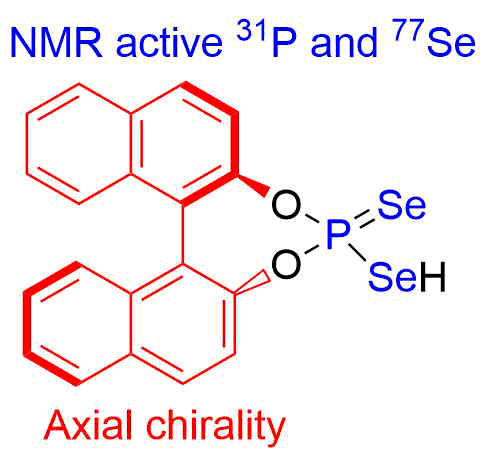
- Synthesis of chiral selenophosphoric acids and study of their catalytic activity ( in collaboration with the University of Regensburg).
Quantum Chemistry
Quantum chemistry methods are based on the principles of quantum mechanics applied to molecular systems. The solution of the quantum chemical problem is reduced to the numerical solution of the Schrödinger equation using various approximations. Modern methods require significant computational resources, therefore calculations are carried out using powerful computational clusters.
Spectroscopy (IR, NMR) and quantum chemistry methods are complement each other. Calculations are used to explain experimentally observed phenomena, to predict experimental results, or to model impossible experiments.
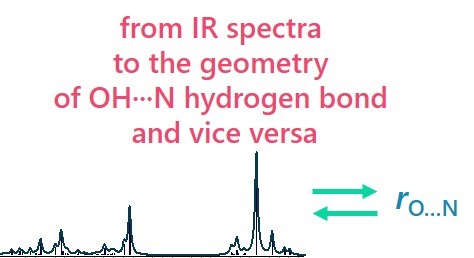
In our laboratory, we solve the problem of finding correlations between the spectral parameters of complexes with hydrogen bonds and their geometry and energy.
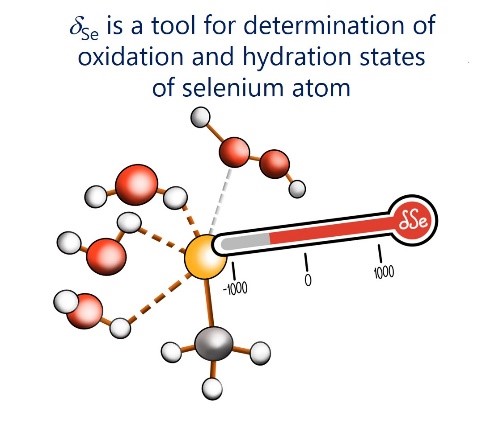 |
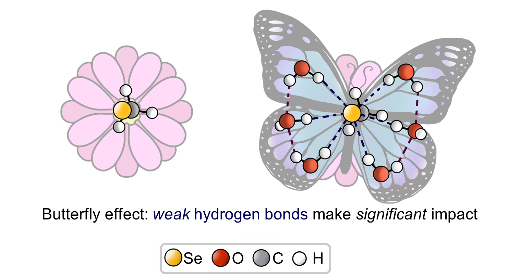 |
 |
| Tupikina et al. Phys. Chem. Chem. Phys, 2021 | Karpov et al. J. Comput. Chem. 2021 | Tupikina et al. Spectrochim. Acta A 2022 |
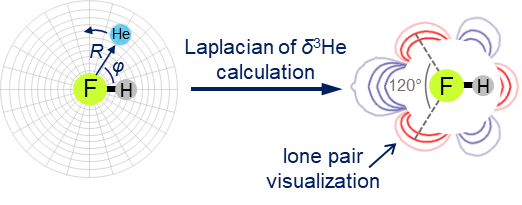
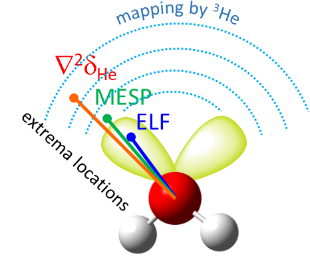
We have proposed a new method for quantum-chemical diagnostics of the features of the outer electronic shell of atoms, molecules and complexes with hydrogen bonds. The keystone of this approach is usage of the 3He atom as a probe particle. Our method allows visualization of both proton-acceptor and proton-donor properties of molecular systems and has advantages over conventional methods (MESP, ELF).
 |
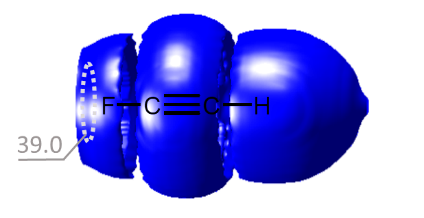 |
 |
| Tupikina et al. J. Phys. Chem. A 2017 | Tupikina et al. J. Comput. Chem. 2018 | Tupikina et al. J. Comput. Chem. 2020 |
One of the directions of quantum-chemical research in our laboratory is the study of the features of the electronic shells of fragments of biological molecules (amino acids, proteins, enzymes). In particular, prediction of possible directions of nucleophilic attack, study of the influence of additional hydrogen bonds with solvent molecules or side chains of a biological molecule on its reactivity.
NMR Spectroscopy
The nuclear magnetic resonance technique (NMR) is based on the registration of resonant absorption of radio frequency energy by a sample placed in a uniform magnetic field. Radiation is absorbed by nuclei with a nonzero magnetic moment (1H, 13C, 19F, 31P, etc.). NMR spectroscopy is the most important method for determining the structure of organic molecules.
In our laboratory, experimental NMR spectroscopy is used to study the properties of various systems with hydrogen and halogen bonds. In particular, we are working on the development of a methodology for interpreting the experimental values of 31P chemical shifts as descriptors of the energy and geometry of the hydrogen bond. Establishing quantitative relationships between the 31P chemical shift is complicated (and therefore becomes more curious :)) due to the fact that the phosphorus nucleus is extremely sensitive to many additional factors.
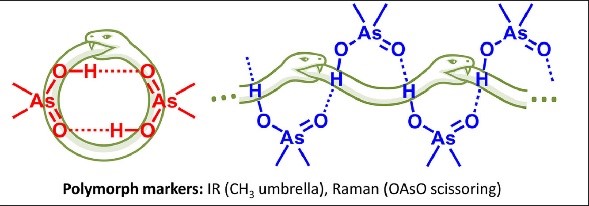 |
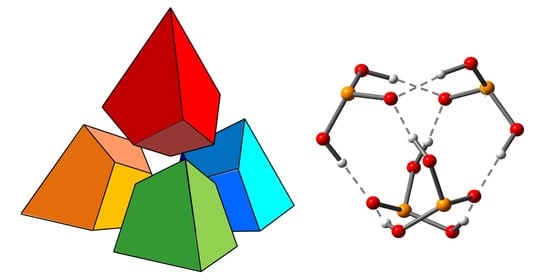 |
 |
| Mulloyarova et al. J. Molec. Struct. 2021 | Giba et al. Symmetry, 2021 | Yakubenko et al. Phys. Chem. Chem. Phys. 2022 |
To obtain high-resolution NMR spectra at low temperatures (up to 100 K), we use and refine a unique technique – we use mixtures of liquefied freon gases as solvents. Under such conditions, the processes of proton and molecular exchange are slowed down and signals of complexes of various stoichiometric and isotopic compositions are resolved on the NMR spectra.
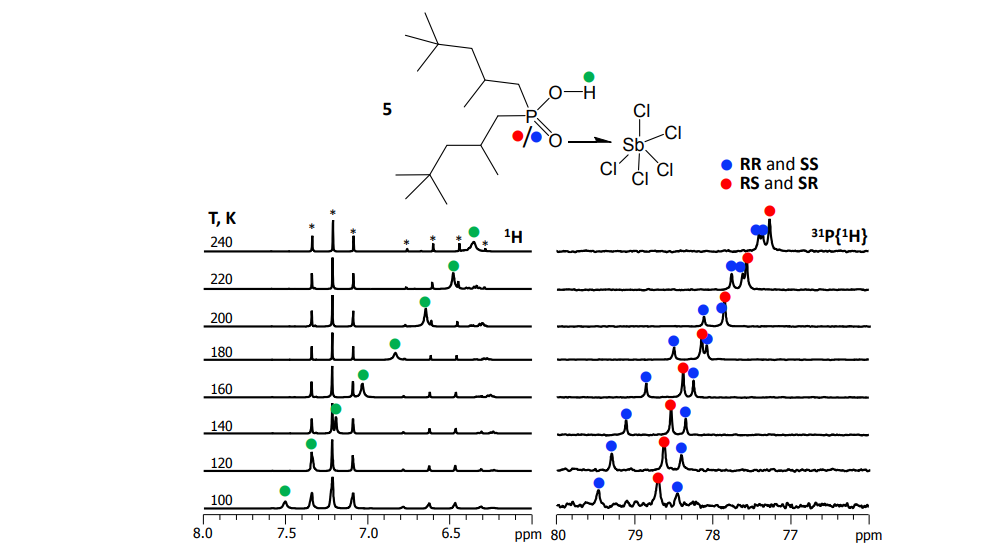
Mulloyarova et al. Phys. Chem. Chem. Phys. 2018
Our grants
- RSF grant23-13-00095 «Non-covalent interactions with high charge cooperativity» (Prof. Dr. P. Tolstoy).
- RSF grant 24-73-10155«Influence of nuclear dynamics on non-covalent interactions: from fundamental description in model associations to prediction of properties of supramolecular systems»(Dr.E.Tupikina)
- RSF grant 24-73-00030 «C-H activation of azine heterocycles under conditions of cooperative catalysis» (Dr. D.Tonkoglazova).
Publications
| D.A. Shitov, M.V. Kaplanskiy, E.Yu. Tupikina, « Influence of the Fe(II) Spin State on Iron-Ligand Bonds in Heme Model Iron-Porphyrin Complexes with 4, 5 and 6 Ligands», ChemPlusChem, 2024. DOI: 10.1002/cplu.202400550. | |
| In this work heme models with four [Fe(II)(P)], five [Fe(II)(P)Im], [Fe(II)(P)(Im)O2] and six ligands [Fe(II)(P)(Im)O2], where P = porphyrin, with different spin states (ms =5, 3 and 1) of the iron atom were investigated using relativistic-corrected quantum chemistry methods (PW6B95-D3-DKH/jorge-TZP-DKH). Dependence of the iron-ligand bond properties on (i) spin state and (ii) number of ligands were analyzed using natural bond orbital analysis, electron density topology, electrostatic potential and electron localization function. It is shown that reversible binding of O2 is possible in case of formation of semicoordination bond between Fe(II) and imidazole. Binding of the fifth and sixth ligand from the energetic and orbital points of view is more favorable for the triplet Fe(II) state. At the same time for the six-coordinated complex [Fe(II)(P)(Im)O2] interconversion of Fe(II) electrons of valent 3d orbital from quintet to triplet and vice versa is possible under thermal fluctuations (energy barriers less than 2 kcal/mol). | |
| A.S. Zakharov, D.V. Krutin, P.O. Mosalyov, E.Yu. Tupikina, A.S. Antonov, P.M. Tolstoy, V.V. Mulloyarova, “Phosphine Selenides: Versatile NMR Probes for Analyzing Hydrogen OH···Se and Halogen I···Se Bonds”, Physical Chemistry Chemical Physics, 2024, published online. DOI: 10.1039/D4CP01895H | |
| Nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for studying the structure and dynamics of various non-covalent interactions. However, often spectral parameters that are applicable for estimation of parameters of one type of non-covalent interaction will be inapplicable for another. Therefore, researchers are compelled to use spectral parameters that are specifically tailored to the type of non-covalent interaction being studied. This complexity makes it difficult to compare different types of non-covalent interactions with each other and, consequently, to establish a strict unified classification for them. This pioneering study proposes to use phosphine selenides as universal probes for investigating hydrogen and halogen bonding in solution. The study was carried out using the example of triethylphosphine selenide Et3PSe complexes with hydrogen bonds of Se⋯HO type and R3PSe (where R: Me, Et, n-Bu, t-Bu and Ph) with halogen bonds of Se⋯X type (where X: I and Br) in solution. The presence of non-covalent interactions was confirmed experimentally by means of 1H, 31P and 77Se NMR, as well as by quantum chemical calculation methods (optimization: PW6B95-D3/def2-QZVP; NMR: B97-2/pcsSeg-2). | |
|
M.V. Kaplanskiy, M.L. Kruglov, A.A. Vanin, E.Yu. Tupikina, “Dynamic of non-covalent interactions during the P–O bond cleavage by ribonuclease A”, Phys. Chem. Chem. Phys., 2024, published online. DOI: 10.1039/D4CP01888E |
|
| In this work, an atomistic-scale investigation of the phosphodiester P–O bond cleavage reaction by the enzyme ribonuclease A was carried out using computer simulation techniques. It is shown that during the reaction the network of non-covalent interactions in the active center of the ribonuclease changes significantly, while the role of these non-covalent interactions is different: coordination of the corresponding groups, electron density transfer, and ligand holding in the active center. It is shown that the process of proton transfer from Asp121 to His119 is the first stage of this reaction; at the same time, the hydrogen bond between the phosphate ligand and the imino group of Arg39 is broken, which, although keeping the ligand in the active center, does not allow the ligand to orient itself more conveniently for subsequent proton transfers. Furthermore, the key step of this reaction occurs: proton transfer with the participation of imidazole rings His12 and His119, in which the guiding role is played by several hydrogen bonds with the participation of Phe120, and the role of an electron density carrier is played by the pnictogen bond between the oxygen of the phosphate ligand and the pyridine-like nitrogen of the imidazole ring His119, which was detected for the first time. | |
| M.A. Kostin, O. Alkhuder, L. Xu, D.V. Krutin, R.E. Asfin, P.M.Tolstoy, “Complexes of phosphine oxides with substituted phenols:hydrogen bond characterization based on shifts of P=O stretching bands”,Phys. Chem. Chem. Phys. 2024, 26, 10234-10242. DOI: 10.1039/D3CP05817D. | |
| In this work IR spectral characteristics of PO groups are used to evaluate the strength of OHO hydrogen bonds. Three phosphine oxides: triphenylphosphine oxide, tributylphosphine oxide and hexamethylphosphoramide are investigated as proton acceptors. The results of the experimental IR study and DFT calculation of 30 complexes formed by phosphine oxides with various substituted phenols or CF3CH2OH in CCl4 solution at room temperature are reported. We show that the PO vibrational frequency changes non-linearly upon hydrogen bond formation and strengthening and that the shift of the PO band could be used for the estimation of hydrogen bond strength in complexes with phosphine oxides. The accuracy of these estimations and the influence of solvation effects on the main characteristics of complexes are discussed. | |
|
D.V. Krutin, A.S. Zakharov, E.Yu. Tupikina, V.V. Mulloyarova, “Unveiling the Electronic Structure Peculiarities of Phosphine Selenides as NMR Probes for Non-covalent Interactions: An Experimental and Theoretical Study”, Phys. Chem. Chem. Phys.,2024, published online. DOI: 10.1039/D4CP01191K |
|
| In this work, R3PSe (R = Me, Et, n-Bu, t-Bu and Ph) were studied experimentally using NMR spectroscopy in solution and the solid-state in combinaton with quantum chemical methods. The study shows that the NMR parameters of these phosphine selenides, such as δP, δSe, and 1JPSe, are sensitive to subtle changes in the electronic environment of the P and Se atoms. Consequently, phosphine selenides R3PSe can serve as promising spectral probes for the detection and quantitative investigation of various non-covalent interactions. Additionally, the variations of R in phosphine selenides influence the observed NMR spectral parameters, primarily through effects such as π-backdonation and hyperconjugation, which have been observed experimentally and confirmed theoretically. | |
| E.Yu. Tupikina, M.V. Sigalov, O. Alkhuder, P.M. Tolstoy, “Charge Relay Without Proton Transfer: Coupling of Two Short Hydrogen Bonds via Imidazole in Models of Catalytic Triad of Serine Protease Active Site”, ChemPhysChem,2024, published online. DOI: 10.1002/cphc.202300970. | |
| A homologous series of 20 substituted alcohol-imidazole-acetate model complexes imitating the charge relay system in Ser-His-Asp catalytic triad of serine proteases is considered quantum-chemically. We show qualitatively that the geometries of alcohol-imidazole and imidazole-acetate short hydrogen bonds are strongly coupled via the central imidazole and such complexes are capable of effectively relaying the charge from acetate to alcohol moiety upon relatively small concerted proton displacements. We hypothesize an alternative catalytic mechanism of serine proteases that does not require two complete proton transfers or hydrogen bond breakage between Ser and His residues. | |
| D. A. Shitov, D. V. Krutin, E. Y. Tupikina, “Mutual influence of non-covalent interactions formed by imidazole: A systematic quantum-chemical study”, J. Comput. Chem., 2024, 45(13), 1046-1060. DOI: 10.1002/jcc.27309. | |
| Imidazole is a five-membered heterocycle that is part of a number of biologically important molecules such as the amino acid histidine and the hormone histamine. Imidazole has a unique ability to participate in a variety of non-covalent interactions involving the NH group, the pyridine-like nitrogen atom or the π-system. For many biologically active compounds containing the imidazole moiety, its participation in formation of hydrogen bond NH⋯O/N and following proton transfer is the key step of mechanism of their action. In this work a systematic study of the mutual influence of various paired combinations of non-covalent interactions (e.g., hydrogen bonds and π-interactions) involving the imidazole moiety was performed by means of quantum chemistry (PW6B95-GD3/def2-QZVPD) for a series of model systems constructed based on analysis of available x-ray data. It is shown that for considered complexes formation of additional non-covalent interactions can only enhance the proton-donating ability of imidazole. At the same time, its proton-accepting ability can be both enhanced and weakened, depending on what additional interactions are added to a given system. The mutual influence of non-covalent interactions involving imidazole can be classified as weak geometric and strong energetic cooperativity—a small change in the length of non-covalent interaction formed by imidazole can strongly influence its strength. The latter can be used to develop methods for controlling the rate and selectivity of chemical reactions involving the imidazole fragment in larger systems. It is shown that the strong mutual influence of non-covalent interactions involving imidazole is due to the unique ability of the imidazole ring to effectively redistribute electron density in non-covalently bound systems with its participation. | |
|
S.A. Meshalkin, S.V. Tsybulin, V.G. Bardakov, I.A. Tatarinov, D.A. Shitov, E.Yu. Tupikina, M.M. Efremova, A.S. Antonov, “Buttressing Effect" in the Halogen-Lithium Exchange in ortho-bromo-N,N-dimethylanilines and Related Naphthalenes”, Chem. Eur. J. 2023, e202303956. DOI: 10.1002/chem.202303956. |
|
| Non-covalent interactions such as coordination of an organolithium reagent by a directing group and steric repulsion of substituents strongly affect the halogen-lithium exchange process. Here we present the manifestation of the “buttressing effect” – an indirect interaction between two substituents issued by the presence of a third group – and its influence on the ease and selectivity of the bromine-lithium exchange and the reactivity of formed aryllithiums. The increase of the size of the “buttressing” substituent strongly affects the conformation of a NMe2 group, forcing it to hinder ortho-bromine and thus slowing down the exchange. In naphthalene substrates bearing two bromines, this suppresses regioselectivity of the reaction. The “buttressing effect” forces formed aryllithiums to deaggregate, thus boosting their reactivity. This facilitates the decomposition via protolisys by ethereal solvents even at low temperatures and in some cases initiates fast Wurtz-Fittig coupling. | |
| M.V. Kaplanskiy, V.V. Karpov, E.Yu. Tupikina, A.S. Antonov, “NMR Descriptors of the Strained Metallacycles Formation in Organo-lithiums: Theoretical Study”, Organic and Biomolecular Chemistry 2023. DOI: 10.1039/d3ob01916k. | |
| For the first time through quantum chemistry methods, the effective use of 1JCLi spin–spin coupling constants as descriptors for assessing the formation of strained metallacycles is demonstrated. Both acyclic organolithiums and 3- to 7-membered metallacycles are examined. 80 organolithium compounds, including both monomeric and dimeric species, with ligands containing fluorine, nitrogen, oxygen, and carbon (in the form of carbanions), are tested. In general, the 1JCLi values below 12 Hz for monomeric species and below 6 Hz for dimeric species serve as clear indicators of strained monomeric metallacycle formation (for 6Li nuclei). The primary contributor to the overall 1JCLi value is the Fermi-contact term, which correlates directly with the carbon-lithium interatomic distance and allows to distinguish between dimers and monomers. | |
| A. A. Yakubenko, E. Yu. Tupikina, A. S. Antonov, “The Role of Conjugation in the Halogen‐Lithium Exchange Selectivity: lithiation of 4, 6, 7, 9‐tetrabromo‐1, 3‐dimethyl‐2, 3‐dihydro‐1H‐perimidine”, Chemistry–A European Journal2023, 29, e202301439. DOI: 10.1002/chem.202301439 | |
| The first case of successful suppression of the coordination of a lithium atom with a dialkylamino group by the effective conjugation of the latter with the aromatic core has been discovered. This effect controls regioselectivity of the bromine−lithium exchange in 4,6,7,9-tetrabromo-1,3-dimethyl-2,3-dihydro-1H-perimidine, which leads to products with the most effective conjugation. As a result, the product of this quadruple exchange demonstrates no tendency of the coordination of the NMe groups to neighboring lithium atoms despite the absence of steric restrictions. Experimental results are explained by means of quantum chemical calculations: geometry optimization, natural bond analysis and scans using the modredundant scheme. | |
| E.Yu. Tupikina, V.O. Korostelev, D.V. Krutin, P. M. Tolstoy, “Evolution of vibrational bands upon gradual protonation/deprotonation of arsinic acid H2As(O)OH in media of different polarity”, Physical Chemistry Chemical Physics2023, 25, 8664 - 8675. DOI: 10.1039/D2CP06060D. | |
| This computational work is devoted to the investigation (MP2/def2-TZVP) of the geometry and IR parameters of arsinic acid H2AsOOH and its hydrogen-bonded complexes under vacuum and in media with different polarity. The medium effects were accounted for in two ways: (1) implicitly, using the IEFPCM model, varying the dielectric permittivity (ε) and (2) explicitly, by considering hydrogen-bonded complexes of H2As(O)OH with various hydrogen bond donors (41 complexes) or acceptors (38 complexes), imitating a gradual transition to the As(OH)2+ or AsO2− moiety, respectively. It was shown that the transition from vacuum to a medium with ε > 1 causes the As(O)OH fragment to lose its flatness. The solvent polar medium introduces significant changes in the geometry and IR spectral parameters of hydrogen-bonded complexes too: as the polarity of a medium increases, weak hydrogen bonds become weaker, and strong and medium hydrogen bonds become stronger; in the case of a complex with two hydrogen bonds cooperativity effects are observed. In almost all cases the driving force of these changes appears to be preferential solvation of charge-separated structures. In the limiting case of complete deprotonation (or conversely complete protonation) the vibrational frequencies of νAsO and νAs–O turn into νAs–O(asym) and νAs–O(sym), respectively. In the intermediate cases the distance between νAsO and νAs–O is sensitive to both implicit solvation and explicit solvation and the systematic changes of this distance can be used for estimation of the degree of proton transfer within the hydrogen bond. | |
| D.O. Tolochenko, S.V. Tsybulin, A.A. Yakubenko, E. Yu. Tupikina, A.S. Antonov, “Spontaneous Cyclization of peri-Diiminonaphthalenes Leading to the Formation of Benzo [de] isoquinolines and Stable Benzo [de] isoquinoliniums”, Organic Letters2023, 25, 6, 977–981. DOI: 10.1021/acs.orglett.3c00035. | |
| The interaction of peri-dilithionaphthalenes with organic cyanides was studied. Instead of the expected peri-diimines, the reaction leads to the formation of three types of benzo[de]isoquinolines. Treatment of unsubstituted 1,8-dilithionaphthalene with aromatic nitriles results in the formation of 1-amino-1,3-diaryl-1H-benzo[de]isoquinolines. In contrast, 4,5-dilithio-1,8-bis(dimethylamino)naphthalene gives an aromatic isoquinolonium cation via elimination of ammonia under the same condition. Upon treatment with tert-butylcyanide, both dilithionaphthalenes undergo a transformation to 1-amino-3,4-di-tert-butyl-4H-benzo[de]isoquinolines. The observed reactivity was supported by quantum chemical calculations. | |
2024 г.
- D.A. Shitov, M.V. Kaplanskiy, E.Yu. Tupikina, « Influence of the Fe(II) Spin State on Iron-Ligand Bonds in Heme Model Iron-Porphyrin Complexes with 4, 5 and 6 Ligands», ChemPlusChem (2024). DOI: 10.1002/cplu.202400550.
- A.VRozhkov, E.Yu. Tupikina, K.I. Tugashov, V.Yu. Kukushkin, « Pure Heterometallic Spodium Bonding», CrystEngComm2024. DOI: 10.1039/D4CE00825A.
- A. Paderina, S. Slavova, E. Tupikina, D. Snetkov, E. Grachova, «Aggregation game: changing solid-state emission using different counterions in mono-alkynylphosphoniumPt(II) complexes », Inorganic Chemistry2024. DOI: 10.1021/acs.inorgchem.4c02130.
- A.S. Zakharov, D.V. Krutin, P.O. Mosalyov, E.Yu. Tupikina, A.S. Antonov, P.M. Tolstoy, V.V. Mulloyarova, “Phosphine Selenides: Versatile NMR Probes for Analyzing Hydrogen OH···Se and Halogen I···Se Bonds”, Physical Chemistry Chemical Physics2024. DOI: 10.1039/D4CP01895H
- M.V. Kaplanskiy, M.L. Kruglov, A.A. Vanin, E.Yu. Tupikina, “Dynamic of non-covalent interactions during the P–O bond cleavage by ribonuclease A”, Physical Chemistry Chemical Physics2024. DOI: 10.1039/D4CP01888E
- D.V. Krutin, A.S. Zakharov, E.Yu. Tupikina, V.V. Mulloyarova, “Unveiling the Electronic Structure Peculiarities of Phosphine Selenides as NMR Probes for Non-covalent Interactions: An Experimental and Theoretical Study”, Physical Chemistry Chemical Physics2024. DOI: 10.1039/D4CP01191K
- J. Eder, A.S. Antonov, E.Yu. Tupikina, R.M. Gschwind, “Chiral Diselenophosphoric Acids for Ion Pair Catalysis: A Novel Approach to Enhance Both Proton Donating and Proton Accepting Properties”, Chem. Eur. J. 2024, e202401793. DOI: 10.1002/chem.202401793
- E.Yu. Tupikina, M.V. Sigalov, O. Alkhuder, P.M. Tolstoy, “Charge Relay Without Proton Transfer: Coupling of Two Short Hydrogen Bonds via Imidazole in Models of Catalytic Triad of Serine Protease Active Site”, ChemPhysChem2024. DOI: 10.1002/cphc.202300970.
- D. A. Shitov, D. V. Krutin, E. Y. Tupikina, “Mutual influence of non-covalent interactions formed by imidazole: A systematic quantum-chemical study”, J. Comput. Chem.2024, 45(13), 1046-1060. DOI: 10.1002/jcc.27309.
- A.Yu. Samsonova, A.Yu. Mikheleva, K.M. Bulanin, N.I. Selivanov, A.S. Mazur, P.M. Tolstoy, C.C. Stoumpos, Y.V. Kapitonov, “Internal Vibrations of Pyridinium Cation in One-Dimensional Halide Perovskites and the Corresponding Halide Salts”, Molecules2024, 29, 78. DOI: 10.3390/molecules29010078.
- M.A. Kostin, O. Alkhuder, L. Xu, D.V. Krutin, R.E. Asfin, P.M. Tolstoy, “Complexes of phosphine oxides with substituted phenols: hydrogen bond characterization based on shifts of P=O stretching bands”, Phys. Chem. Chem. Phys.2024, 26, 10234-10242. DOI: 10.1039/D3CP05817D.
- S. Baykova, S. Baykov, K. Geyl, P. Tolstoy, V. Boyarskiy, “N-Pyridylureas as Masked Isocyanates for the Late-Stage Diversification of Pyridine-N-Oxides”, ChemistrySelect2024, 9, e202400720. DOI: 10.1002/slct.202400720.
- В.А. Розенцвет, Н.А. Саблина, Д.М. Ульянова, П.М. Толстой, «Структурная характеристика низкомолекулярного полибутадиена, синтезированного под действием катионной инициирующей системы», Изв. АН, серияхимическая2024, 73(4), 1035-1045.
- V.A. Rozentsvet, N.A. Sablina, D.M. Ulyanova, P.M. Tolstoy, “Structural characterization of low molecular weight polybutadiene synthesized using cationic initiation system”, Russ. Chem. Bull.2024, 73(4), 1035-1045. DOI: 10.1007/s11172-024-4218-6.
- K. Sotiriadis, A.S. Mazur, Peter M. Tolstoy, P. Mácová, A. Viani, “External magnesium sulfate attack on hydrated Portland-limestone cements: Effect of the concurrent presence of sodium chloride in the corrosive environment and metakaolin admixture in the binder”, Cem.Concr. Compos.2024, 151, 105614. DOI: 10.1016/j.cemconcomp.2024.105614.
2022
- A. Yakubenko, A. Puzyk, V. Korostelev, V. Mulloyarova, E. Tupikina, P. Tolstoy, A. Antonov, “Oxidation of triphenylpnictogens and complexation of resulted di-phenylpnictoginic acids in solution and solid state”, Phys. Chem. Chem. Phys. 2022, accepted. DOI: 10.1039/D2CP00286H.
- E. Tupikina, A.A. Titova, M.V. Kaplanskiy, E.R. Chakalov, M.A. Kostin, P.M. Tolstoy, “Estimations of OH···N hydrogen bond length from positions and intensities of IR bands”, Spectrochimica Acta A 2022, accepted. DOI: 10.1016/j.saa.2022.121172
- M.A. Kostin, S.A. Pylaeva, P.M. Tolstoy, “Phosphine oxides as NMR and IR spectroscopic probes for the estimation of the geometry and energy of hydrogen bonds: PO…H-A hydrogen bonds”, Phys. Chem. Chem. Phys. 2022, 24, 7121-7133. DOI: 10.1039/D1CP05939D.
- Aliyarova, I.S., Tupikina, E.Yu., Ivanov, D.M., Kukushkin, V. Yu., “Metal-Involving Halogen Bonding Including Gold(I) as a Nucleophilic Partner. The Case of Isomorphic Dichloroaurate(I)Halomethane Cocrystals”, Inorganic Chemistry 2022, 61, 5, 2558–2567. DOI: 10.1021/acs.inorgchem.1c03482.
- K. Sotiriadis, K. Aspiotis, A. Mazur, P. Tolstoy, E. Badogiannis, S. Tsivilis, “Characterization of Old Concrete from a Heritage Structure of Inousses Cluster of Islands”, Lect. Notes Civ. Eng. 2022, 209, 80-89. DOI: 10.1007/978-3-030-90788-4_7.
- M. Nikishina, L. Perelomov, Y. Atroshchenko, E. Ivanova, L. Mukhtorov, P. Tolstoy, “Sorption of fulvic acids and their compounds with heavy metal ions on clay minerals”, Soil Syst. 2022, 6, 2. DOI: 10.3390/soilsystems6010002.
- V. Rozentsvet, N. Sablina, D. Ulyanova, S. Kostjuk, P. Tolstoy, “Structure of terminal units of polybutadiene synthesized via anionic mechanism”, Polym. Bull. 2022, 79, 1239-1256. DOI: 10.1007/s00289-021-03549-5.
- F. Shakirova, U. Markova, P. Tolstoy, A. Bulatov, A. Shishov, “A new hydrophobic deep eutectic solvent based on thymol and 4-(dimethylamino)benzaldehyde: derivatization and microextraction of urea”, J. Mol. Liquids 2022, 353, 118820. DOI: 10.1016/j.molliq.2022.118820.
- A. Mazur, P. Tolstoy, K. Sotiriadis, “13С, 27Al and 29Si NMR investigation of the hydration kinetics of Portland-limestone cement pastes containing CH3-COO–R+ (R=H or Na)”, Materials 2022, 15, 2004. DOI: 10.3390/ma15062004.
- В.А. Розенцвет, Д.М. Ульянова, Н.А. Саблина, М.Г. Кузнецова, П.М. Толстой, «Полимеризация 1,3-пентадиена под действием катионных каталитических систем на основе алюминийорганических соединений», Известия АН 2022, 4, 787-795.
- V.A. Rozentsvet, D.M. Ulyanova, N.A. Sablina, S.V. Kostjuk, P.M. Tolstoy, I.A. Novakov, “Cationic polymerization of butadiene using alkyl aluminum compounds as co-initiators: an efficient approach toward solid polybutadienes”, Polym. Chem. 2022, 3, 1596-1607. DOI: 10.1039/d1py01684a.
2021
- A.A. Yakubenko, V.V. Karpov, E.Yu. Tupikina, A.S. Antonov, “Lithiation of 2,4,5,7-Tetrabromo-1,8-bis(dimethylamino)naphthalene: Peculiarities of Directing Groups' Effects and the Possibility of Polymetalation”, Organometallics 2021, 40(21), 3627–36368. DOI: 10.1021/acs.organomet.1c00489.
- V. Lyutoev, T. Shumilova, A. Mazur, P. Tolstoy, “NMR spectral characteristics of ultrahigh-pressure high-temperature impact glasses of the giant Kara astrobleme (Pay-Khoy, Russia)”, Minerals 2021, 11, 1418. DOI: 10.3390/min11121418.
- В.А. Розенцвет, Н.А. Саблина, Д.М. Ульянова, П.М. Толстой, И.А. Новаков, «Полимеризация изопрена под действием катионных каталитических систем на основе триэтилалюминия», Доклады РАН 2021, 499, 66-70. DOI: 10.31857/S2686953521040063. V.A. Rozentsvet, N.A. Sablina, D.M. Ulyanova, P.M. Tolstoy, I.A. Novakov, “Polymerization of Isoprene Using Cationic Catalytic Systems Based on Triethylaluminum”, Doklady Phys Chem. 2021, 499, 73-76. DOI: 10.1134/S0012501621080017.
- E.Yu. Tupikina, P.M. Tolstoy, A.A. Titova, M.A. Kostin, G.S. Denisov, “Estimations of FH···X hydrogen bond energies from IR intensities: Iogansen's rule revisited”, J. Comput. Chem. 2021, 41. DOI: 10.1002/jcc.26482
- E.Y. Tupikina, S.G. Yastrebov, , “Molecular Complexes of Glycine with Cations H+, Ca2+, and Phosphine Oxide H3PO”, Technical Physics Letters 2021, 47(2), 147–149. DOI: 10.1134/S1063785021020140.
- I.S. Giba, P.M. Tolstoy, “Self-assembly of tetrahedral hydrogen-bonded cage tetramers of phosphonic acid”, Symmetry 2021, 13, 258. DOI: 10.3390/sym13020258.
- I.S. Giba, V.V. Mulloyarova, G.S. Denisov, P.M. Tolstoy, “Sensitivity of 31P NMR chemical shifts to hydrogen bond geometry and molecular conformation for complexes of phosphinic acids with pyridines”, Magn. Reson. Chem. 2021, 59(4), 465–477. DOI: 10.1002/mrc.5123.
- V.V. Mulloyarova, A.M. Puzyk, A.A. Efimova, A.S. Antonov, R.A. Evarestov, I.S. Aliyarova, R.E. Asfin, P.M. Tolstoy, “Solid-State and Solution-State Self-Association of Dimethylarsinic Acid: IR, NMR and Theoretical Study”, J. Mol. Struct. 2021, 1234, 130176. DOI: 10.1016/j.molstruc.2021.130176.
- В.А. Розенцвет, Н.А. Саблина, Д.М. Ульянова, С.Н. Смирнов, П.М. Толстой, «Строение полимерной цепи поли-1,3-пентадиена, синтезированного на стереоспецифической каталитической системе», Известия АН 2021, 4, 773-779. V.A. Rozentsvet, N.A. Sablina, D.M. Ulyanova, S.N. Smirnov, P.M. Tolstoy, “Structure of the polymer chain of poly(1,3-pentadiene) synthesized using a stereospecific catalytic system”, Russ. Chem. Bull. 2021, 70(4), 773-779. DOI: 10.1007/s11172-021-3150-2.
- B. Koeppe, J. Guo, P.M. Tolstoy, H.-H. Limbach, “NMR and UV-vis spectroscopic studies of models for the hydrogen bond system in the active site of photoactive yellow protein: hydrogen bond cooperativity and medium effects”, J. Phys. Chem. B 2021, 125, 22, 5874-5884. DOI: 10.1021/acs.jpcb.0c09923.
- Ł. Hetmańczyk, P. Szklarz, A. Kwocz, M. Wierzejewska, M. Pagacz-Kostrzewa, M.Ya. Melnikov, P.M. Tolstoy, A. Filarowski, “Polymorphism and Conformational Equilibrium for Nitro-Acetophenone in the Solid State and Under the Matrix Condition”, Molecules 2021, 26, 3109. DOI: 10.3390/molecules26113109.
- E.Yu. Tupikina, V.V. Karpov, P.M. Tolstoy, “On the influence of water molecules on the outer electronic shells of R–SeH, R–Se(–) and R–SeOH fragments in selenocysteine amino acid residue”, Phys. Chem. Chem. Phys. 2021, 23, 13965-13970. DOI: 10.1039/D1CP01345A.
- J. Savosina, M. Agafonova-Moroz, M. Khaydukova, A. Legin, V. Babain, P. Tolstoy, D. Kirsanov, “On the radiolytic stability of potentiometric sensors with plasticized polymeric membranes”, Chemosensors 2021, 9, 214. DOI: 10.3390/chemosensors9080214.
- E. Bulatov, T. Eskelinen, A. Ivanov, P. Tolstoy, E. Kalenius, P. Hirva, M. Haukka, “Noncovalent axial I∙∙∙Pt∙∙∙I interactions in platinum(II) complexes strengthen in the excited state”, ChemPhysChem 2021, 22, 1-7. DOI: 10.1002/cphc.202100468.
- Ł. Hetmańczyk, E.A. Goremychkin, J. Waliszewski, M.V. Vener, P. Lipkowski, P.M. Tolstoy, A. Filarowski, “Spectroscopic identification of hydrogen bond vibrations and quasi-isostructural polymorphism in N-salicylideneaniline”, Molecules 2021, 26, 5043. DOI: 10.3390/molecules26165043.
- V. Karpov, A. Puzyk, P. Tolstoy, E. Tupikina, “Hydration of selenolate moiety: ab initio investigation of properties of O–H···Se(–) hydrogen bonds in CH3Se(–)∙∙∙(H2O)n clusters”, J. Comput. Chem. 2021, 42, 2014-2023. DOI: 10.1002/jcc.26733.
- V. Rozentsvet, N. Sablina, D. Ulyanova, S. Kostjuk, P. Tolstoy, “Structure of terminal units of polybutadiene synthesized via anionic mechanism”, Polym. Bull. 2021, accepted. DOI: 10.1007/s00289-021-03549-5.
2020
- K. Sotiriadis, P. Mácová, A.S. Mazur, A. Viani, P.M. Tolstoy, S. Tsivilis, “Long-term thaumasite sulfate attack on Portland-limestone cement concrete: A multi-technique analytical approach for assessing phase assemblage”, Cement and Concrete Research 2020, 130, 105995. DOI: 10.1016/j.cemconres.2020.105995.
- N.M. Kuznetsov, S.I. Belousov, A.V. Bakirov, S.N. Chvalun, R.A. Kamyshinsky, A.A. Mikhutkin, A.L. Vasiliev, P.M. Tolstoy, A.S. Mazur, E.D. Eidelman, E.B. Yudina, A.Ya. Vul', “Unique rheological behavior of detonation nanodiamond hydrosols: the nature of sol-gel transition”, Carbon 2020, 161, 486-494. DOI: 10.1016/j.carbon.2020.01.054.
- A.Yu. Baranov, A.S. Berezin, D.G. Samsonenko, A.S. Mazur, P.M. Tolstoy, V.F. Plyusnin, I.E. Kolesnikov, A.V. Artem'ev, “New Cu(I) halide complexes showing TADF combined with room temperature phosphorescence: the balance tuned by halogens”, Dalton Trans. 2020, 49, 3155-3163. DOI: 10.1039/D0DT00192A.
- I. Kritskiy, T. Volkova, S. Ivanov, A. Mazur, P. Tolstoy, I. Terekhova, “Methotrexate-Loaded Metal-Organic Frameworks on the Basis of γ-Cyclodextrin: Design, Characterization, in Vitro and in Vivo Investigation”, Materials Science and Engineering C 2020, 111, 10774. DOI: 10.1016/j.msec.2020.110774.
- V.А. Rozentsvet, N.А. Sablina, D.M. Ulyanova, P.M. Tolstoy, S.N. Smirnov, I.A. Novakov, “Identification of the structure of polybutadiene terminal units by NMR spectroscopy with T2-filter”, Doklady Phys. Chem. 2020, 491, 40-42. DOI: 10.1134/S0012501620040028.
- V. Rozentsvet, N. Sablina, D. Ulyanova, M. Kuznetsova, S. Kostjuk, P. Tolstoy, “A new approach towards investigation of structure of terminal units of poly(1,3-pentadiene) using T2-filtered NMR spectroscopy”, Macromol. Chem. Phys. 2020, 2000168. DOI: 10.1002/macp.202000168.
- A.S. Antonov, V.V. Karpov, E.Yu. Tupikina, P.M. Tolstoy, M.A. Vovk, “Aggregation Behavior of Lithionaphthalenes in Solution: Experimental and Theoretical Study”, Organometallics 2020, 39, 3705. DOI: 10.1021/acs.organomet.0c00524.
- A.S. Antonov, E.Yu. Tupikina, V.V. Karpov, V.V. Mulloyarova, V.G. Bardakov, “Sterically Facilitated Intramolecular Nucleophilic NMe2 Group Substitution in the Synthesis of Fused Isoxazoles: Theoretical Study”, Molecules, 2020, 25, 5977. DOI: 10.3390/molecules25245977.
- A.S. Antonov, V.G. Bardakov, V.V. Mulloyarova, “Sterically facilitated meta-lithiation of arenes, containing electron-donating groups”, J. Org. Chem. 2020, 906, 121068. DOI: 10.1016/j.jorganchem.2019.121068
- A.A. Antonov, A.A. Yakubenko, “Noncovalent Li···H Interaction in the Synthesis of peri-Disubstituted Naphthalene Proton Sponges”, Synthesis 2020; 52, 98-104. DOI: 10.1055/s-0039-1690230
- E. Yu. Tupikina, G. S. Denisov, A. S. Antonov, P. M. Tolstoy, “Unusual behaviour of the spin-spin coupling constant 1JCH upon formation of CH•••X hydrogen bond”, Phys. Chem. Chem. Phys. 2020, 22, 1994-2000. DOI: 10.1039/C9CP05964D
- E. Yu. Tupikina, K. G. Tokhadze, G. S. Denisov, P. M. Tolstoy, “Lone pairs mapping by Laplacian of 3He NMR chemical shift”, J. Comput. Chem. 2020, 41, 1194-1199. DOI: 10.1002/jcc.26166.
- E.Yu. Tupikina, K.G. Tokhadze, V.V. Karpov, G.S. Denisov, P.M. Tolstoy, “Stretching force constants as descriptors of energy and geometry of F···HF hydrogen bonds”, Spectrochim. Acta A 2020, 241, 118677. DOI: 10.1016/j.saa.2020.118677.
- A.S. Mazur, M.A. Vovk, P.M. Tolstoy, “Solid-state NMR of carbon nanostructures (graphite, graphene, carbon nanotubes, nanodiamonds, fullerene) in 2000-2019: a mini-review”, Fuller. Nanotub. Car. N. 2020, 28(3), 202-213. DOI: 10.1080/1536383X.2019.1686622.
- A.S. Ostras’, D.M. Ivanov, A.S. Novikov, P.M. Tolstoy, “Phosphine oxides as spectroscopic halogen bond descriptors: IR and NMR correlations with interatomic distances and complexation energy”, Molecules 2020, 25, 1406. DOI: 10.3390/molecules25061406.
- V.Y. Mikshiev, A.F. Pozharskii, A. Filarowski, A.S. Novikov, A.S. Antonov, P.M. Tolstoy, M.A. Vovk, O.V. Khoroshilova, “ How strong hydrogen bond with amide nitrogen atom is?”, ChemPhysChem, 2020, 21, 651-658. DOI: 10.1002/cphc.201901104.
- V.V. Mulloyarova, D.O. Ustimchuk, A. Filarowski, P.M. Tolstoy, «H/D Isotope Effects on 1H NMR Chemical Shifts in Cyclic Heterodimers and Heterotrimers of Phosphinic and Phosphoric Acids», Molecules 2020, 25, 1907. DOI: 10.3390/molecules25081907.
- K. Jóźwiak, A. Jezierska, J.J. Panek, E.A. Goremychkin, P.M. Tolstoy, I.G. Shenderovich, A. Filarowski, “Inter- vs. intra-molecular hydrogen bond patterns and proton dynamics in phthalic acid associates”, Molecules 2020, 25, 4720. DOI: 10.3390/molecules25204720.
- V. Torres-Barthelemy, N. Pérez-Hernández, I.G. Shenderovich, P.M. Tolstoy, G.S. Denisov, H.-H. Limbach, “NMR-detected Host-Guest Proton Exchange as a novel Tool to study Surface/Volume Ratios and Filling of Cavities of mesoporous Solids”, J. Phys. Chem. C 2020, 124(40), 22082-22095. DOI: 10.1021/acs.jpcc.0c04889.
- P. Piękoś, A. Jezierska, J. Panek, E.A. Goremychkin, A. Pozharskii, A. Antonov, P. Tolstoy, A. Filarowski, “Symmetry/asymmetry of NHN hydrogen bond in protonated 1,8-bis(dimethylamino)naphthalene”, Symmetry 2020, 12, 1924. DOI: 10.3390/sym12111924.
- S.N. Yunusova, D.S. Bolotin, M.A. Vovk, P.M. Tolstoy, V.Yu. Kukushkin, “Tetrabromomethane as an Organic Catalyst: a Kinetic Study of CBr4-Catalyzed Schiff Condensation”, Eur. J. Org. Chem. 2020, 6763-6769. DOI: 10.1002/ejoc.202001180.
- D.L. Melnikova, Z.F. Badrieva, M.A. Kostin, C. Maller, M. Stas, A. Buczek, M.A. Broda, T. Kupka, A.-M. Kelterer, P.M. Tolstoy, V.D. Skirda, “On Complex Formation Between 5-Fluorouracil and beta-Cyclodextrin in Solution and in the Solid State: IR Markers and Detection of Short-Lived Complexes by Diffusion NMR”, Molecules 2020, 25, 5706. DOI: 10.3390/molecules25235706.
Information for students
The Laboratory welcomes 1st-4th year students for graduation works and research works in the following areas:
- NMR spectroscopy (Prof. Dr. Peter M. Tolstoy, room 2150, This email address is being protected from spambots. You need JavaScript enabled to view it.)
- Organometallic chemistry (Dr.DariaI. Tonkoglazova,This email address is being protected from spambots. You need JavaScript enabled to view it.; Dr. Semyon V. Tsybulin, This email address is being protected from spambots. You need JavaScript enabled to view it., room 2135)
- Quantum chemistry (Dr. Elena Yu. Tupikina, room 2123)
