Colloid Chemistry
Thermodynamics, surface and electrosurface properties of colloid nanosystems
Team
Team Leader
 |
Anatoly Ivanovich RusanovAcademician of Russian Academy of Sciences, Professor, Doctor of Science in Chemistry, Head of the department of Colloid Chemistry The author of 800 articles and 11 monographs. Aworded by Mendeleev and Rehbinder Golden Medals of thr Russian Academy of Sciences Topics:
|
Team Members
 |
Liudmila Eduardovna ErmakovaProfessor, Doctor of Science in Chemistry Topics:
This email address is being protected from spambots. You need JavaScript enabled to view it., This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Nikolai Gennad'evich SukhodolovAssociate Professor, Doctor of Science in Chemistry Topics:
This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Aleksandr A. VaninSenior lecturer, Philosophy Doctor in Chemistry Topics:
This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Anna Valerievna VolkovaAssociate Professor, Philosophy Doctor in Chemistry Topics:
This email address is being protected from spambots. You need JavaScript enabled to view it. |
Associate Members
 |
Elena Nikolaevna BrodskayaLeading researcher, Doctor of science in Physical and Mathematical science Topics: the structure and status of nanosystems, including surface layers of fluids within the statistical theory and computer simulation. This email address is being protected from spambots. You need JavaScript enabled to view it., This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Evgenia Victorovna GolikovaDoctor of Science in Chemistry Topics:
This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Anatoly Nikolaevich ZhukovProfessor, Doctor of Science in Chemistry Topics:
This email address is being protected from spambots. You need JavaScript enabled to view it. |
Research Topics
1. Development of thermodynamics of nanosystems. Thermodynamic and colloidal-chemical characteristics of surfactant solutions and micellar systems.
Thermodynamic, kinetic and molecular modeling of micelles and processes in micellar systems Development of thermodynamics and kinetics of micelle formation in non-polar media. Formulation of the mass action law for reverse micelles. Study of the role of water in the formation of reverse micelles. Study of the solubilization of macromolecules in solutions of surfactants and micellar systems. (RFBR grant 20-03-00641 2020-2022 «Development of thermodynamics and kinetics of micelle formation in nonpolar media»).
2. The structure and status of nanosystems, including surface layers of fluids and micellar systems within the statistical theory and computer simulation.
Computer simulation of molecular systems by molecular dynamics and Monte Carlo methods. The objects of research are
- micellar solutions of ionic and non-ionic surfactants,
- multicomponent two-phase adsorption systems. Changes in droplet shape and molecular mobility of hydrocarbons in a porous medium are studied.
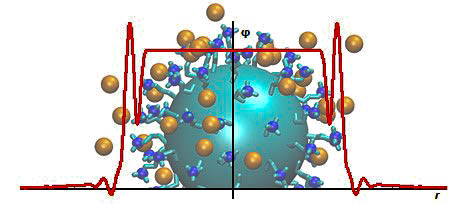
Fig. 1. Electrical field around a direct spherical micelle.
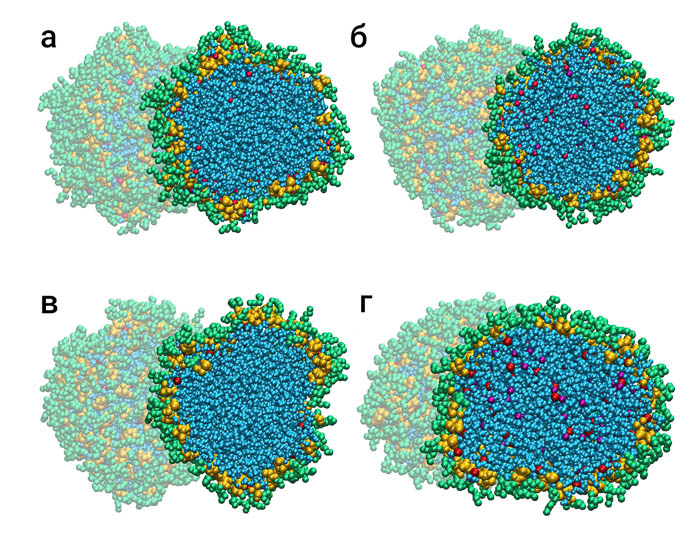
Fig. 2. reversed micelles: а) AOTNa-H2O-C8H18; б) +NaCl; в) (AOT)2Ca-H2O-C8H18; г) +CaCl2.
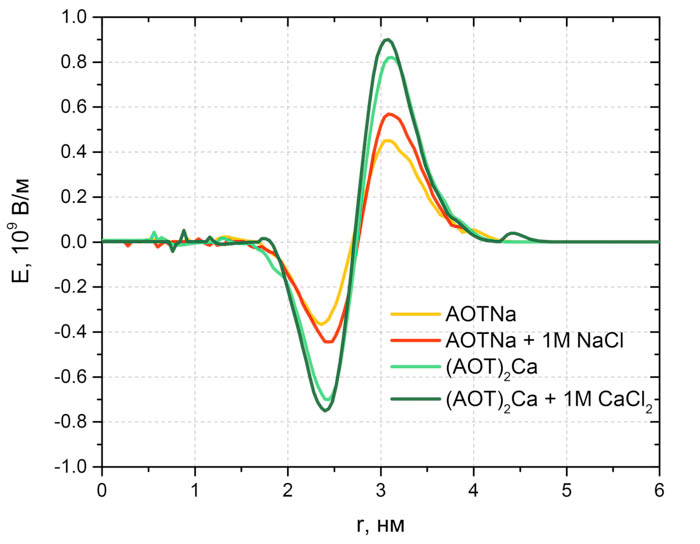
Fig. 3. Distribution of local electrical potential in reverse micelles with AOTNa and AOT2Ca with and without salt.
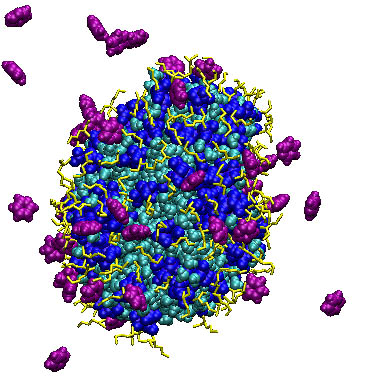
The second problem in modeling of reverse micelles is the study of the dependence of solubilization on the molecular structure of solubilisant.
 |
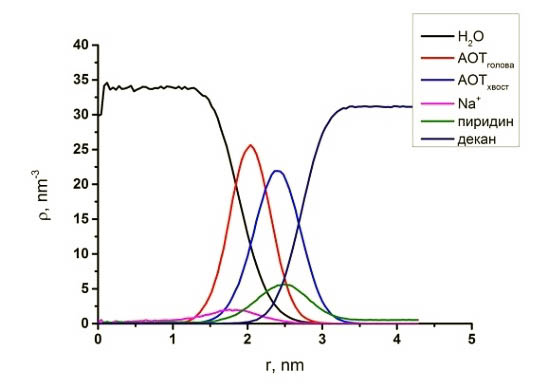 |
Fig. 4. Reverse micelle of АОТ and local profiles of partial densities in the water- decane system with pyridine.
When the water-carbon mixture is adsorbed in slit-shaped pores, depending on the composition of the mixture and the absorption field, aggregates of different shapes are formed, which determines the transport characteristics of the systems.
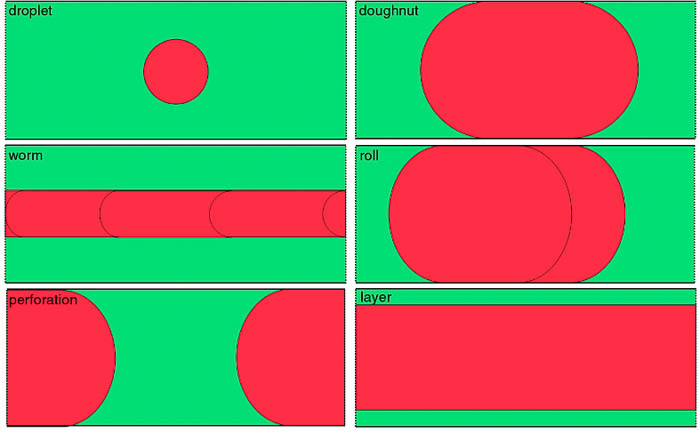
Fig. 5. Six possible types of aggregates in a slit containing a non-wetting component in a mixture.
3. Electrosurface phenomena in macro- and nanodisperse systems (the structure of the electrical double layer at dielectric–electrolyte solution interface, adsorption and electrokinetic characteristics of disperse materials).
Research is devoted to the experimental and theoretical study of the electrical double layer (EDL) structure at the solid-liquid interface (dielectric–electrolyte solution), the adsorption and electrokinetic characteristics of disperse materials in indifferent and containing specifically sorbed ions electrolytes solutions in a wide range of pH and ionic strength of solutions, as well as the calculation of the electrochemical parameters of the interface: surface reaction constants, ion adsorption potentials and EDL potentials. The objects of study are both free-dispersed systems – aqueous dispersions of a number of simple (SiO2, Al2O3, CeO2, ZrO2, TiO2, Y2O3 etc.) and composite (complex oxides MgAl2O4, Y3Al5O12 and particles with the "core-shell" structure: TiO2/SiO2, Al2O3/SiO2, etc.) nanoparticles, and capillary-porous bodies (high-silica porous glasses and polymer membranes).
4. Aggregation stability of nanodisperse systems.
Theoretical and experimental study of stability, coagulation and heterocoagulation processes of free-dispersed oxide systems in electrolyte solutions, regularity of volume changes in structuring colloidal nanosystems
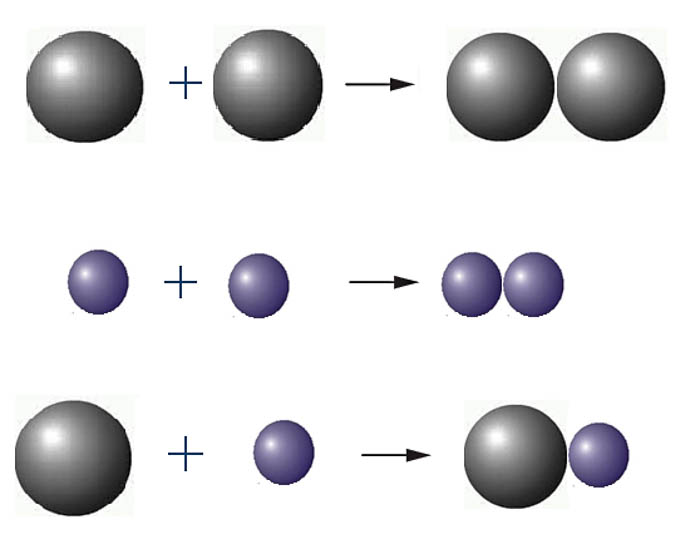
Fig. 6. Coagulation and heterocoagulation of particles in binary system.
5. The investigation of equilibrium and transport characteristics of channel nanostructures in electrolyte solutions.
Study of equilibrium (structural parameters, surface charge, electrokinetic potential) and transport (electrical conductivity, ion transport numbers, filtration potential) characteristics of membrane systems (mainly membranes made of high-silica porous glasses (PGs) and composites based on them) in various electrolyte solutions. The work is carried out in cooperation with the Institute of Chemistry of the Silicates of the Russian Academy of Sciences.
6. Preparation of simple and composite oxide materials (nanoparticles, membranes, ceramics) by sol-gel synthesis and molecular layering.
The sol-gel method is a modern low-temperature and relatively cheap method for obtaining modern materials (nanoparticles, coatings, ceramics) of various composition, morphology and dispersion, as well as shapes and sizes (in the case of bulk ceramics) at atmospheric pressure, which makes it possible to "fine" control over the chemical composition of the obtaining material.
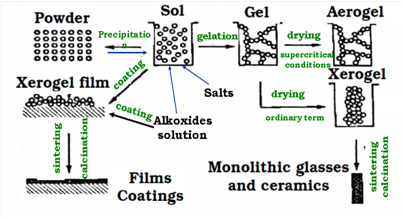
Fig. 7. The concept of obtaining various materials, coatings and powders by the methods of sol-gel technology.
At present, the focus of our research is the synthesis of polycrystalline structural and optical alumina ceramics, YAG-ceramics based on yttrium-aluminum garnet, and AMS-ceramics based on aluminum-magnesium spinel, those are important applications, by the sol-gel technology methods along with the study of their functional properties
The molecular layering method from the gas phase makes it possible to synthesize highly organized ultrathin films of various compositions and functional properties on the substrate surface, setting the required coating thickness by the number of reaction cycles.
In our research work, high-silica porous glasses (PG) of various composition and dispersion are used as a matrix for obtaining composite materials (sorbents, membranes, photocatalysts). PG is a product of leaching of heat-treated alkali borosilicate glasses. At the same time, porous glasses are not only an inert substrates but also excellent sorbents and selective membranes capable of regeneration.
Modification of porous glasses is carried out by:
- Addition of modifier during the production of the initial two-phase glass
In this way, for example, ferromagnetic iron-containing PGs were obtained and their colloidal-chemical properties in electrolyte solutions were studied (RFBR grant 17-03-01011 "Preparation and study of structural and electrosurface properties of ferromagnetic porous glasses" (2017–2019), project leader L.E. Ermakova). Porous glasses containing the magnetite phase (Fe3O4) can be used in microelectronics, in the creation of sensor devices, spintronics, as magnetic membranes and sorbents.
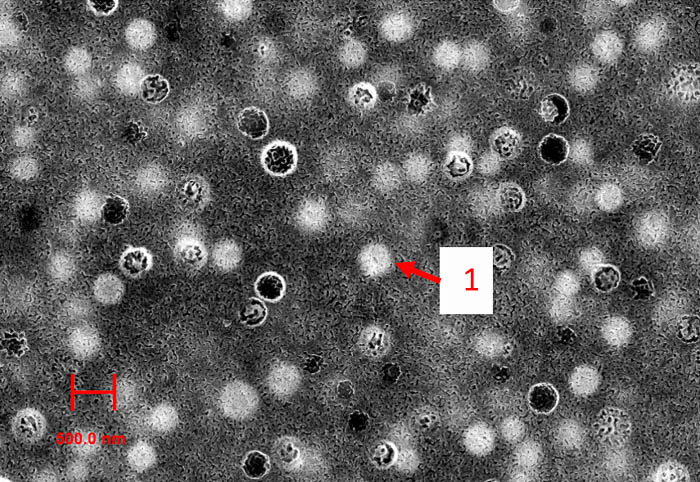
Fig. 8. SAM image of the surface of iron-containing PG with inclusions of magnetite (spherical areas of type 1)
- Modification of the outer and inner surfaces of the PG using molecular layering, sol-gel technology, chemical vapor deposition
Titanium dioxide was chosen as a modifier, which possesses such properties as low toxicity, efficiency and high photocatalytic activity (studies were started within the framework of the RFBR grant 14-03-01062 "Synthesis, colloidal-chemical and photocatalytic properties of composite oxide nanosystems "high-silica porous glass-titanium dioxide "(2014–2016), project leader A.V. Volkova). The obtained composites can be used as a new type of separating membranes, sorbents, photocatalysts.
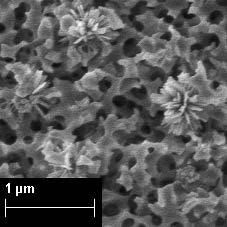
Fig. 9. SEM image of porous glass modified with titanium oxide.
- The introduction of nanoparticles and various chemical compounds into the porous space of the PG.
Impregnation of high-silica porous matrices with salts of various metals (silver halides, tin salts, zinc salts, etc.) with subsequent additional treatment (temperature, laser, etc.) is currently the most common method for obtaining both porous nanocomposites and quartzoid nanostructured materials. The obtained composites can be used for photonics, laser technology, optical instrumentation, nanobiotechnology, for the creation of sensors, including biosensors, electrodes for lithium batteries, solar cells, photodetectors. (RFBR grant 20-03-00544 "Electrosurface characteristics of high-silica porous glasses and quartz-like glasses doped by metallic compounds" (2020–2022), project leader L.E. Ermakova).
Investigation of the functional properties of porous glasses and their composites
Study of the sorption processes of organic and multiply charged inorganic ions onto porous sorbents. Investigation of the photocatalytic processes of decomposition of organic dyes in an aqueous medium.
7. Langmuir-Blodgette films and development of new methods of the production of sorbents based on these films.
The method of obtaining Langmuir-Blodgett (PLB) films makes it possible to vary their properties by changing the structure of the polar part of the amphiphilic molecule, the composition of the monolayer, solution (subphase), and the conditions of transfer to a solid substrate. The uniqueness of the structures obtained by the Langmuir-Blodgett method has led to their use in both basic and applied research. Langmuir-Blodgett films or systems obtained by dispersing collapsed stearic acid monolayers removed from the surface of an aqueous subphase containing Fe (III) ions have a surface that predominantly consists of iron ions reliably bound to the surface of a solid. This made it possible to use them as metal-affinity sorbents of high selectivity and efficiency.
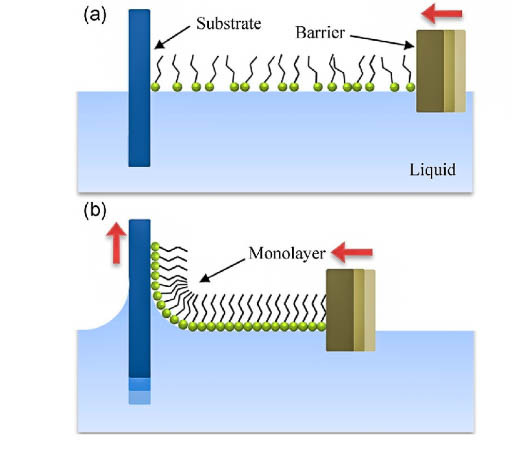
Fig. 10. The mechanism for obtaining Langmuir-Blodgett films.
8. Physical and chemical properties of Langmuir-Blodgett films containing quantum dots of different nature.
It is of interest to obtain LBPs containing quantum dots. Quantum dots exhibit unique optical and electronic properties, which make them a promising material for applications in a variety of fields, from use in optoelectronic devices to use as markers in chemical and immunoassay. In particular, quantum dots have narrow fluorescence peaks, the position of which depends on the size of nanocrystals, broad absorption spectra, and high photostability. The production of thin films with a uniform distribution of quantum dots will expand the area of their application.
Publications
- Volkova A.V., Ermakova L.E., Golikova E.V. Peculiarities of coagulation of the γ-Al2O3 hydrosol in NaCl solutions. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 516 (2017) 129–138.
- Vanin A.A., Brodskaya E.N. Simulation study of influence of component polarizability on the properties of the electric double layer of an ionic micelle. Colloids and Surfaces A: Physicochemical and Engineering Aspects (2017) 522, 58–65.
- Kopanichuk I.V., Vanin A.A., Brodskaya E.N. Disjoining pressure and structure of a fluid confined between nanoscaleю. Colloids and Surfaces A(2017) 527, 42–48.
- Rusanov A.I. On the Problem of Determining Aggregation Numbers from Surface Tension Measurements. Langmuir. Volю 33, Is. 44. P. 12643-12650.
- Rusanov A.I., Shchekin A.K., Volkov N.A. Diffusion in micellar systems: theory and molecular modeling. Russian Chemical Reviews. Vol. 86 (2017). P. 567-588.
- Rusanov A.I. Theory of surfactant diffusion in micellar systems with variable aggregation numbers. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018. Vol. 551. P. 158-164.
- Ermakova L.E., Kuznetsova A.S., Volkova A.V., Antropova T.V. Structural and electrosurface properties of iron-containing nanoporousglasses in KNO3 solutions. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2019. Vol. 576. P. 91-102.
- Rusanov A.I., Brodskaya E.N. Dispersion forces in nanoscience. Russian Chemical Reviews, 2019, 88 (8) 837 – 874.
- Kopanichuk I.V., Novikov V.A., Vanin A.A., Brodskaya E.N. The electric properties of AOT reverse micelles by molecular dynamics simulations. Journal of molecular liquids. 2019. Vol. 296.
- Kopanichuk I.V., Berezhnaya A.S., Sizova A.A., Vanin A.A., Sizov V.V., Brodskaya E.N. The shape of the liquid-liquid interface for oil/water mixtures in slit pores. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2020. Vol. 601. DOI: 10.1016/j.colsurfa.2020.124884
