Department of Organic Chemistry
Rooms 3186 (office), 3193 (lab) 4135 (lab)
Noncovalent Organocatalysis
Short URL for media go.spbu.ru/rgbolotin
In the past decade, organocatalysis has been the focus of extensive studies owing to its significant advantages over catalysis by metal-containing species including lower toxicity, reduced environmental footprint, and low to negligible sensitivity to air and moisture. In general, organocatalysts can function either through a covalent or noncovalent bonding activation. A covalent activation mode involves the formation of covalent bond(s) between a substrate and catalyst, whereas a noncovalent mode involves the activation of substrates through noncovalent linkages to the catalyst. For noncovalent catalysis, an organic catalyst typically interacts with a substrate through hydrogen bonding, whereas catalytic reactions involving halogen or chalcogen bonding are far less explored.

Group Members
 |
Group leader Bolotin Dmitrii S.Full professor This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Il’in Mikhail V.Researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Sysoeva Alexandra A.Junior researcher This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Putnin Ivan O.Graduate Student |
|
|
Pirogova Tatyana A.undergraduate student This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Safinskaya Yana V.Research assistant This email address is being protected from spambots. You need JavaScript enabled to view it. |
Research Interests
Study of noncovalent oranocatalysis is a complex task requiring complementary research in the following scientific fields:
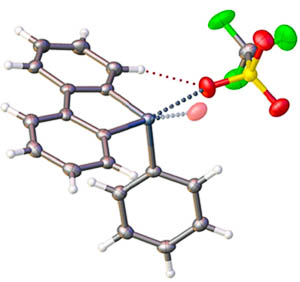 |
Synthetic organic and organoelement chemistry. Our group is focused on rational design of organoelement species exhibiting high catalytic activity in transformations of organic and coordination compounds. Compounds of our general interest are cationic heterocycles featuring exo- and endocyclic halogen or chalcogen atoms, as well as urea and squaramide derivatives. |
 |
Physical organic chemistry. Study of catalytic activity includes determination of kinetic and thermodynamic parameters of the studied model reactions. This research is performed using spectroscopic techniques (NMR, IR, UV-Vis) and thermal analyses. Determination of influence of electronic and steric factors on kinetic and thermodynamic parameters of the reactions allows us to conclude plausible mechanisms of reactions and mechanisms of the catalysis. |
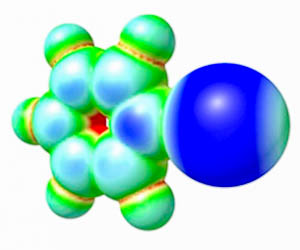 |
Theoretical calculations. Plausible mechanisms of catalysis are typically confirmed (or rejected) by us using DFT calculations. Theoretical calculations are also performed before the synthetic work to shed light on the most promising compounds, which should exhibit the highest catalytic activity. |
Current Grants
 Grant # 103922061 New Generation Organocatalysts as a Replacement for Metal-containing Lewis Acids for Chemical and Pharmaceutical Industries (Bolotin D.S.).
Grant # 103922061 New Generation Organocatalysts as a Replacement for Metal-containing Lewis Acids for Chemical and Pharmaceutical Industries (Bolotin D.S.).
 Grant #23-23-00091 New Catalytic Systems Based on Bifunctional Complexes of Late 3d-metals Featuring Halogen Bond-Donating Ligands (Il'in M.V.).
Grant #23-23-00091 New Catalytic Systems Based on Bifunctional Complexes of Late 3d-metals Featuring Halogen Bond-Donating Ligands (Il'in M.V.).
 Grant #23-73-10003 Organocatalysis Based on sigma-hole carriers for Transition to Ecological Energy- and Resource-saving Processes (Bolotin D.S.).
Grant #23-73-10003 Organocatalysis Based on sigma-hole carriers for Transition to Ecological Energy- and Resource-saving Processes (Bolotin D.S.).
Recent Publications
2024
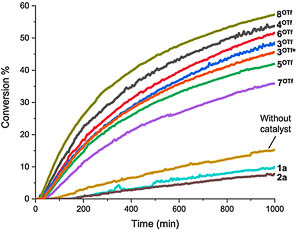
I. O. Putnin, A. A. Sysoeva, M. V. Il’in, D. S. Bolotin, Iodonium and Telluronium Triflates Serving as Noncovalent Organocatalysts Provide Catalytic Effect in the Schiff Condensation Due to Different Reasons, ChemCatChem, 2024, e202400672. DOI: 10.1002/cctc.202400672.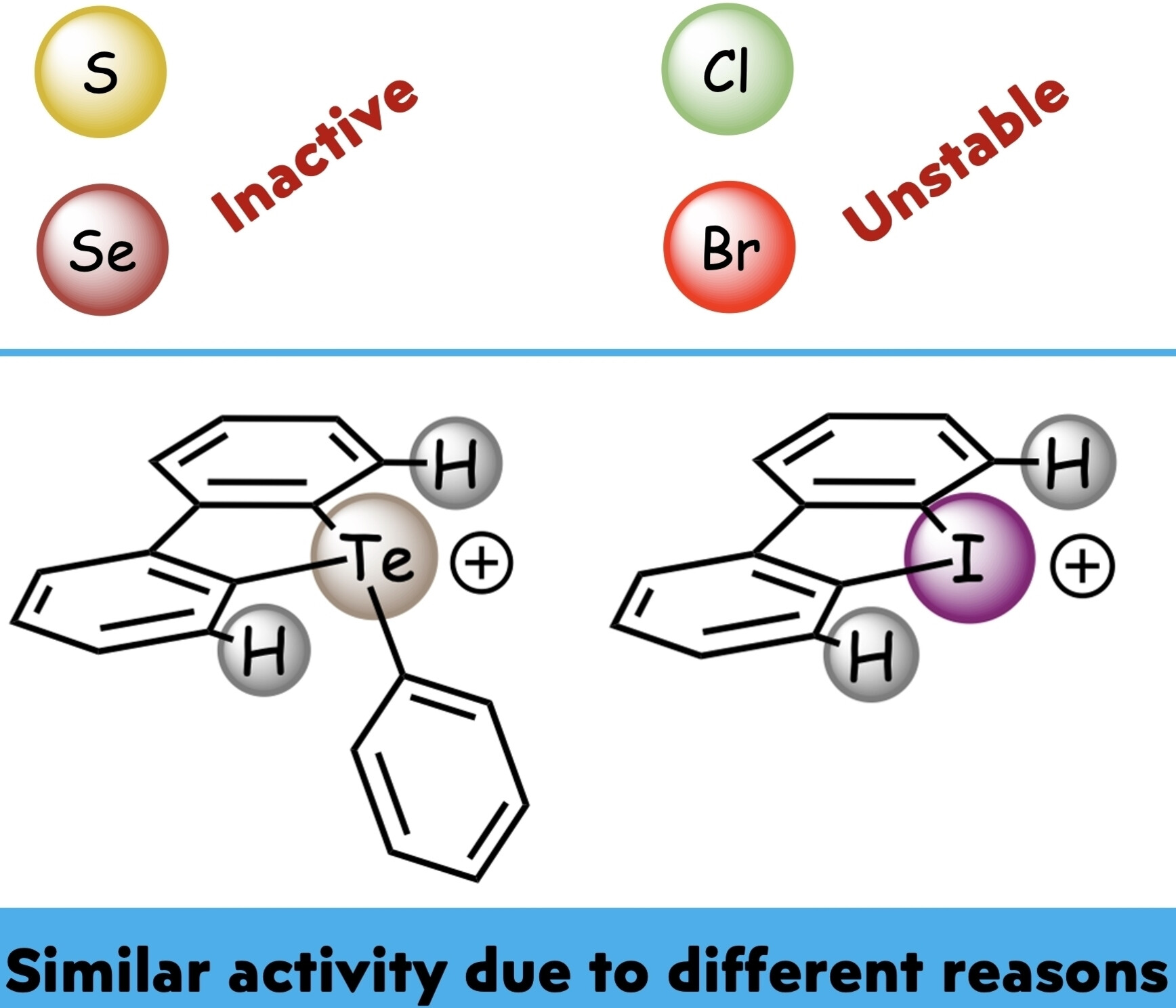
Sulfonium, selenonium, telluronium triflates, as well as chloro- nium, bromonium, and iodonium triflates have been examined in the model Schiff condensation as chalcogen- and halogen bond donating organocatalysts, respectively. The kinetic data indicated that the catalytic effect of the telluronium salt is provided via the decrease of enthalpy of activation of the reaction, whereas the catalytic effect of the iodonium salt –unexpectedly – is caused by the decrease of the value of the entropy of activation. In addition, it was experimentally shown that the catalytic activity of sulfonium and selenonium salts is significantly lower than that of chloronium and bromonium salts, but the latter pair of species is significantly less stable under the reaction conditions than the former pair.
A. A. Sysoeva, M. V. Il’in, D. S. Bolotin,Cooperative Covalent–Noncovalent Organocatalysis of the Knoevenagel Condensation Based on an Amine and Iodonium Salt Mixture, ChemCatChem, 2024, e202301668. DOI: 10.1002/cctc.202301668.
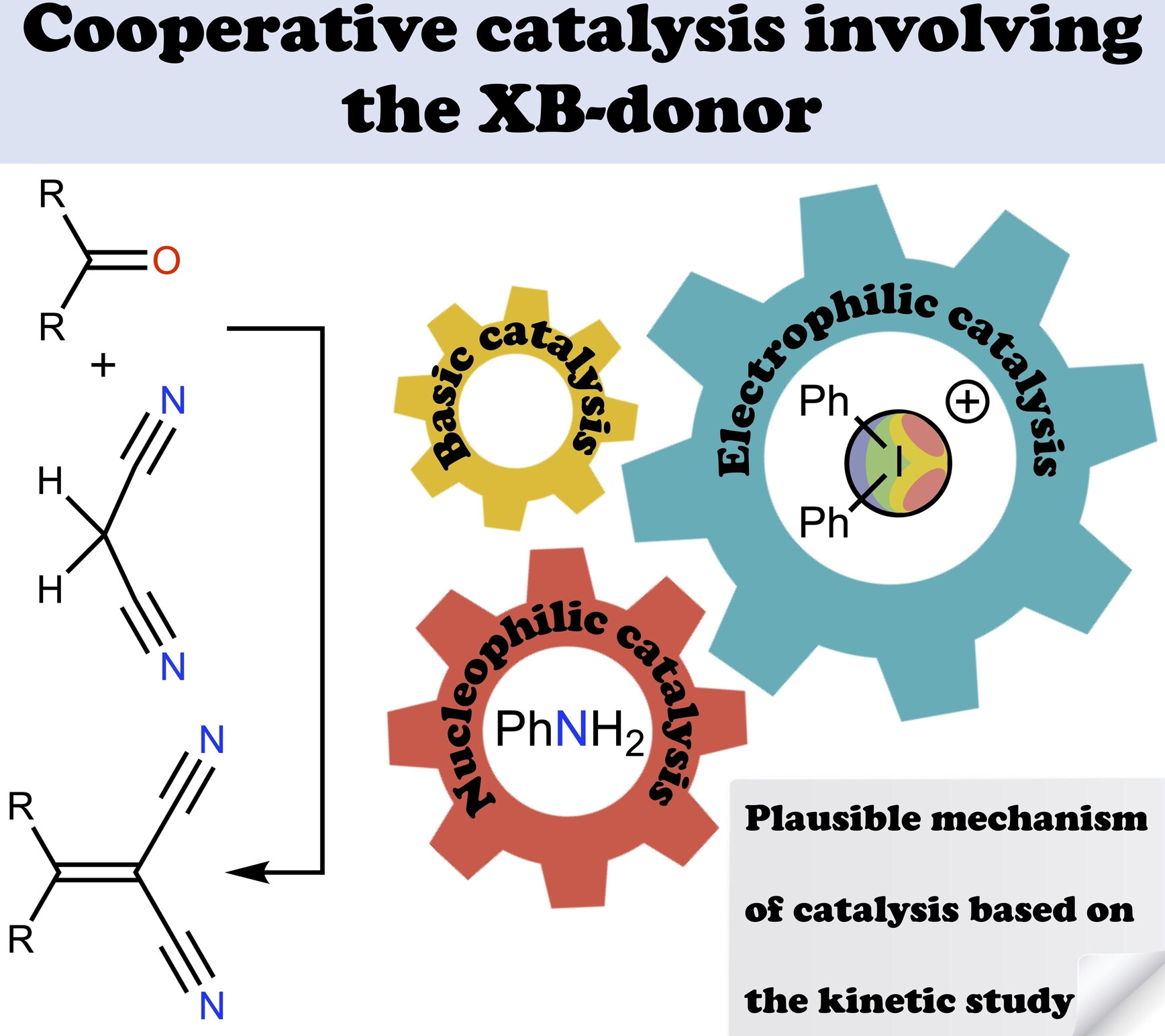
The experimentally obtained kinetic data has indicated the existence of a synergetic cocatalytic effect provided by an iodonium salt in the base-catalyzed Knoevenagel condensation. The diphenyliodonium triflate serving as the halogen bond-donating Lewis acid provides the higher cocatalytic effect than zinc(II) triflate or triflic acid serving, respectively, as the metal cation-based Lewis acid and Brønsted acid. Such a cocatalytic effect remains the same for a broad scope of carbonyl compounds covering aldehydes featuring electron-withdrawing or electron-donating substituents, as well as ketone involved in the reaction.
M. V. Il’in, Ya. V. Safinskaya, D. A. Polonnikov, A. S. Novikov,D. S. Bolotin,Chalcogen- and Halogen-Bond-Donating Cyanoborohydrides Provide Imine Hydrogenation, J. Org. Chem., 2024, 89, 2916. DOI: 10.1021/acs.joc.3c02282.
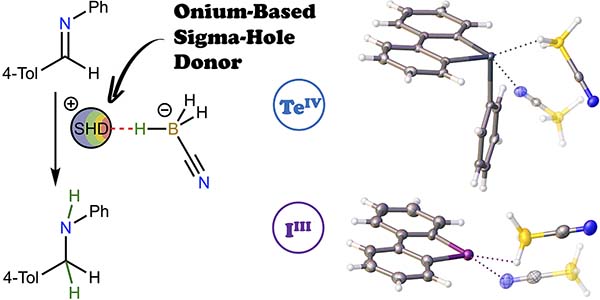
Sulfonium, selenonium, telluronium, and iodonium cyanoborohydrides have been synthesized, isolated, and fully characterized by various methods, including single-crystal X-ray diffraction (XRD) analysis. The quantum theory of atoms in molecules’ analysis based on the XRD data indicated that the hydride···σ-hole short contacts observed in the crystal structures of each compound have a purely noncovalent nature. The telluronium and iodonium cyanoborohydrides provide a significantly higher rate of the model reaction of imine hydrogenation compared with sodium and tetrabutylammonium cyanoborohydrides. Based on the NMR and high-resolution electrospray ionization mass spectrometry data indicating that the reaction progress is accompanied by the cation reduction, a mechanism involving intermediate formation of elusive onium hydrides has been proposed as an alternative to conventional electrophilic activation of the imine moiety by its ligation to the cation’s σ-hole.
A. Sysoeva, A. S. Novikov, M. V. Il’in, D. S. Bolotin, Halogen bonded associates of iodonium salts with 18-crown-6: does structural flexibility or structural rigidity of the s-hole donor provide efficient substrate ligation?, New J. Chem., 2024, 48, 12929. DOI: 10.1039/d4nj02105c.
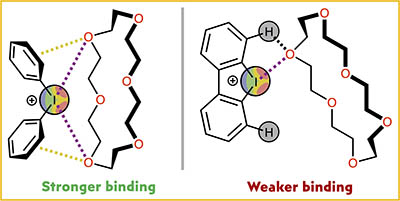
1H NMR titration of 18-crown-6 with diphenyliodonium triflate and dibenziodolium triflate indicated that the acyclic iodine(III)-containing species has a higher binding constant value compared with the cyclic analogue. Formation of triple associates diphenyliodonium×××18-crown-6×××diphenyliodonium was observed in CD3CN. DFT calculations and QTAIM analysis indicated that the acyclic iodonium salt forms a higher number of interactions with the crown ether compared with the cyclic cation, which results in the formation of triple associates. The formation of dibenziodolium×××18-crown-6×××dibenziodolium triple associates turned out to be energetically unfavorable, which agrees with the experimentally obtained data.
S. N. Yunusova, A. S. Novikov, D. S. Bolotin, M. V. Il’in, Iodonium cation stabilizes square-planar configuration of the silver(I) tetratriflate, Inorg. Chim. Acta., 2024, 568, 122079. DOI: 10.1016/j.ica.2024.122079.
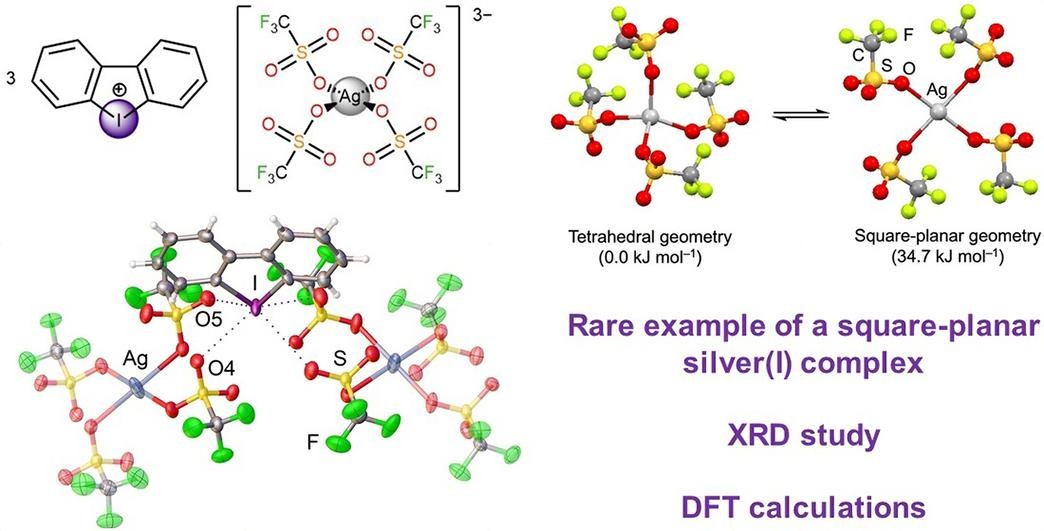
In this work, we have experimentally and theoretically shown that halogen bonding can stabilize the rareexample of square-planar configuration of the silver(I) center. The crystallographic analysis of tris-dibenziodoliumsilver(I) tetratriflate reveals two types of halogen bonding interactions between the triflate ligands and thedibenziodolium cation, which might stabilize the such square-planar coordination geometry. Theoretical calculationsconfirm the noncovalent nature of the I⋅⋅⋅O contacts and provide insights into their electronicproperties.
A. S. Novikov, D. S. Bolotin, M. V. Il’in, Pyrazolyliodoniumplatinum(II) trichloride features the highest positive electrostatic potential on the iodine atom among uncharged halogen bond donors, Inorg. Chim. Acta, 2024, 573, 122335. DOI: 10.1016/j.ica.2024.122335.
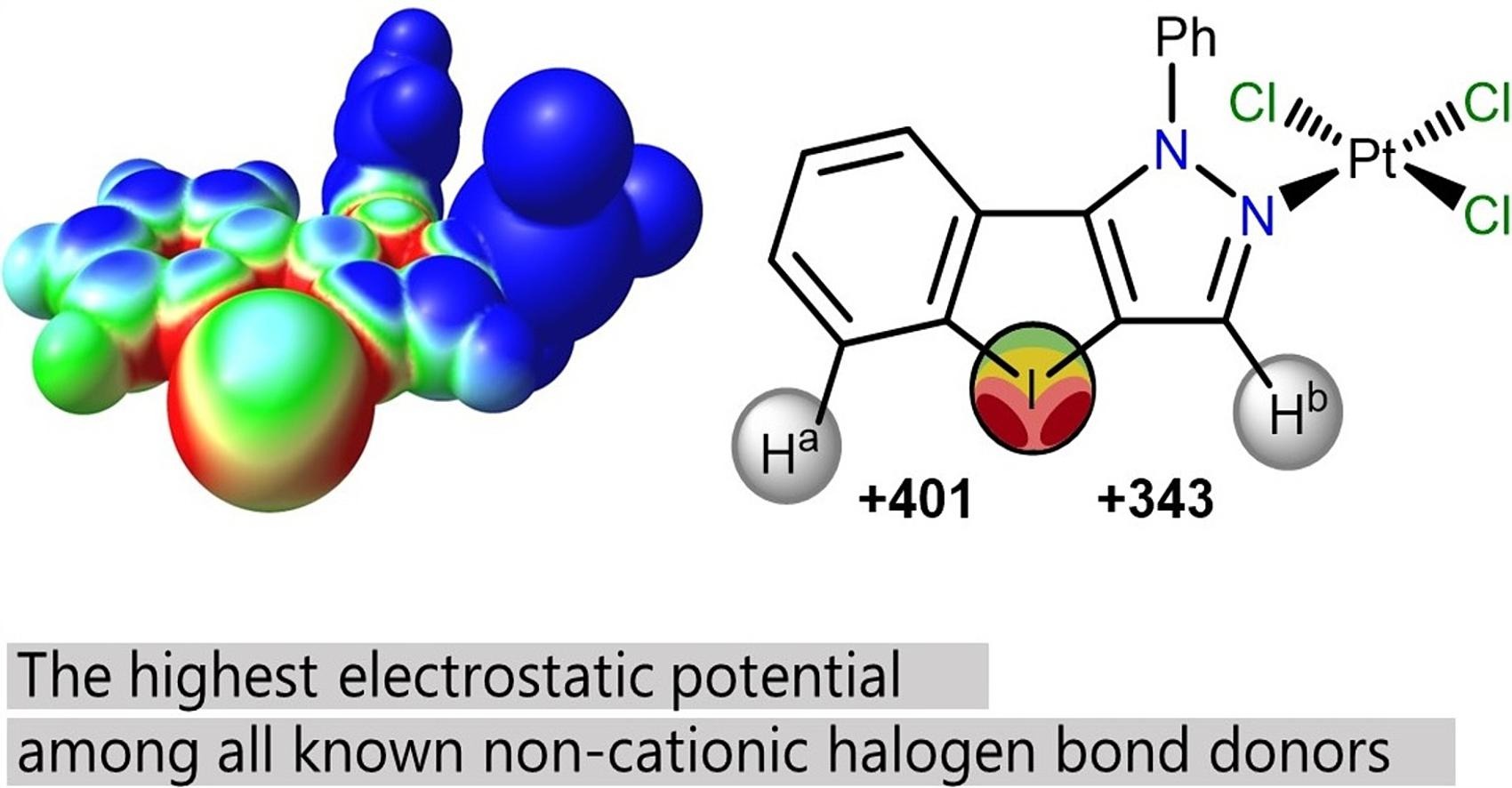
This study presents a new uncharged platinum(II) complex with a pyrazolyliodonium ligand, which features anexceptionally large σ-hole on the iodine atom. Single-crystal X-ray diffraction analysis reveals the structure of the[LPtCl3]⋅2DMF complex, highlighting two types of intermolecular halogen bonds: a C− I⋯O bond with DMF andC−I⋯Cl bonds with the platinum complex. Quantum chemical calculations confirm the high positive electrostaticpotential on the iodine σ-hole, demonstrating its significant halogen bond donor ability.
2023
Sysoeva A.A., Novikov A.S., Suslonov V.V., Bolotin D.S., Il'in M.V., 2,1,3-Benzoselenadiazole-containing zinc(II) halide complexes: Chalcogen bonding in the solid state and catalytic activity in the Schiff condensation, Inorg. Chim. Acta, 2024, 561, 121867. DOI: 10.1016/j.ica.2023.121867.
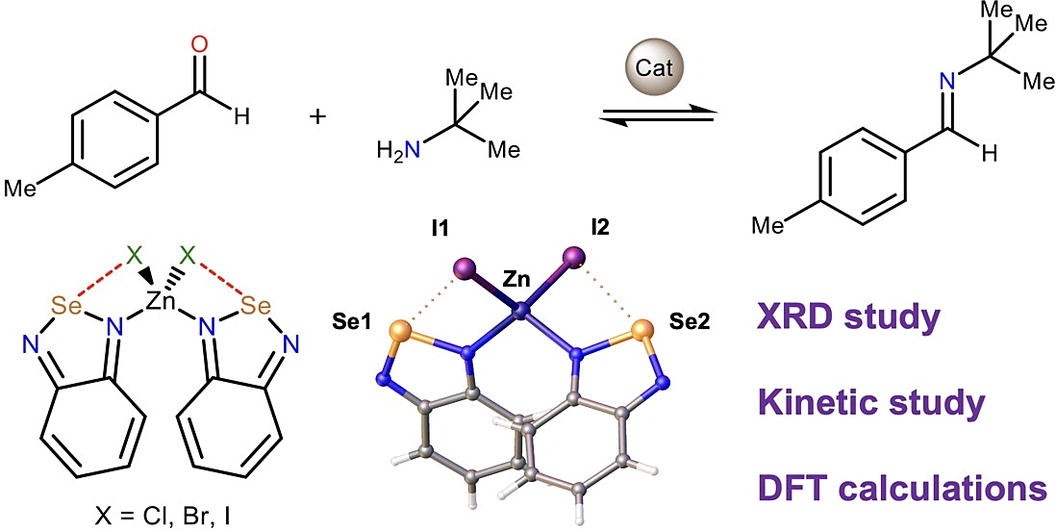 XRD study of crystals of zinc(II) halide complexes featuring 2,1,3-benzoselenadiazole (L) ligands [ZnL2X2] (X=Cl, Br, I) indicated availability of intramolecular chalcogen bonds between the Se atom and the halide ligands, which affect a rotation barrier around the Zn–N bonds and thus control a geometry of these species. In the solid state, the Se atom additionally forms intermolecular chalcogen bonds with the N atom of the 2,1,3-benzoselenadiazole ligands, which results in formation of infinite 1D chains. Theoretical calculations and experimental kinetic study indicated that although the Se atom serves as a Lewis acid, coordination of the organic ligand to the zinc(II) center does not provide enhanced catalytic activity of the latter in the model reaction of the Schiff condensation.
XRD study of crystals of zinc(II) halide complexes featuring 2,1,3-benzoselenadiazole (L) ligands [ZnL2X2] (X=Cl, Br, I) indicated availability of intramolecular chalcogen bonds between the Se atom and the halide ligands, which affect a rotation barrier around the Zn–N bonds and thus control a geometry of these species. In the solid state, the Se atom additionally forms intermolecular chalcogen bonds with the N atom of the 2,1,3-benzoselenadiazole ligands, which results in formation of infinite 1D chains. Theoretical calculations and experimental kinetic study indicated that although the Se atom serves as a Lewis acid, coordination of the organic ligand to the zinc(II) center does not provide enhanced catalytic activity of the latter in the model reaction of the Schiff condensation.
Il’in M.V., Polonnikov D.A., Novikov A.S., Sysoeva A.A., Safinskaya Ya.V., Bolotin D.S., Influence of Coordination to Silver(I) Centers on the Activity of Heterocyclic Iodonium Salts Serving as Halogen-Bond-Donating Catalysts, ChemPlusChem, 2023, 88, e202300304. DOI: 10.1002/cplu.202300304.
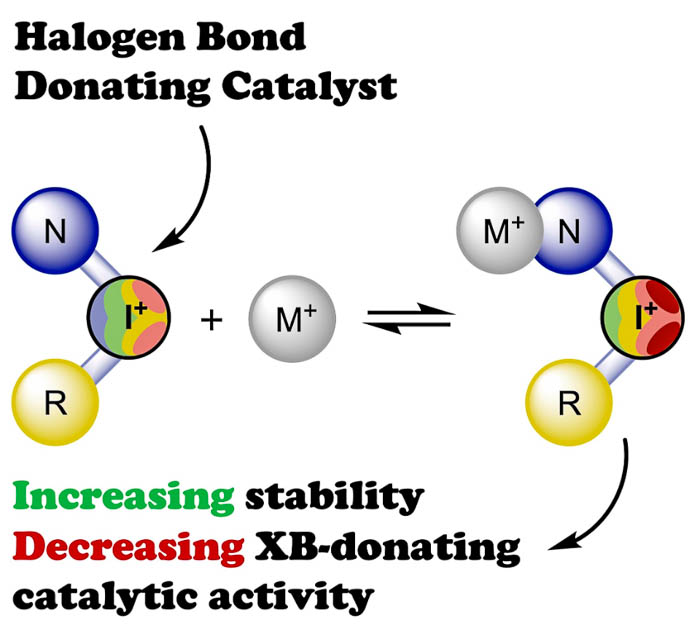 Kinetic data based on the 1H NMR monitoring and computational studies indicate that in a solution pyrazole-containing iodonium triflates and silver(I) triflate bind each other, and such an interplay results in the decrease of the total catalytic activity of the mixture of these Lewis acids compared to the separate catalysis of the Schiff condensation, the imine–isocyanide coupling, or the nucleophilic attack on a triple carbon–carbon bond. Moreover, the kinetic data indicate that such a cooperation with the silver(I) triflate results in prevention of decomposition of the iodonium salts during the reaction progress. XRD study confirms that the pyrazole-containing iodonium triflate coordinates to the silver(I) center via the pyrazole N atom to produce a rare example of a pentacoordinated trigonal bipyramidal dinuclear silver(I) complex featuring cationic ligands.
Kinetic data based on the 1H NMR monitoring and computational studies indicate that in a solution pyrazole-containing iodonium triflates and silver(I) triflate bind each other, and such an interplay results in the decrease of the total catalytic activity of the mixture of these Lewis acids compared to the separate catalysis of the Schiff condensation, the imine–isocyanide coupling, or the nucleophilic attack on a triple carbon–carbon bond. Moreover, the kinetic data indicate that such a cooperation with the silver(I) triflate results in prevention of decomposition of the iodonium salts during the reaction progress. XRD study confirms that the pyrazole-containing iodonium triflate coordinates to the silver(I) center via the pyrazole N atom to produce a rare example of a pentacoordinated trigonal bipyramidal dinuclear silver(I) complex featuring cationic ligands.
Sysoeva A.A., Novikov A.S., Il'in M.V., Bolotin D.S., Solvent-modulated binding selectivity of reaction substrates to onium-based sigma-hole donors, Catal. Sci. Technol., 2023, 13, 3375-3385. DOI: 10.1039/D3CY00071K.
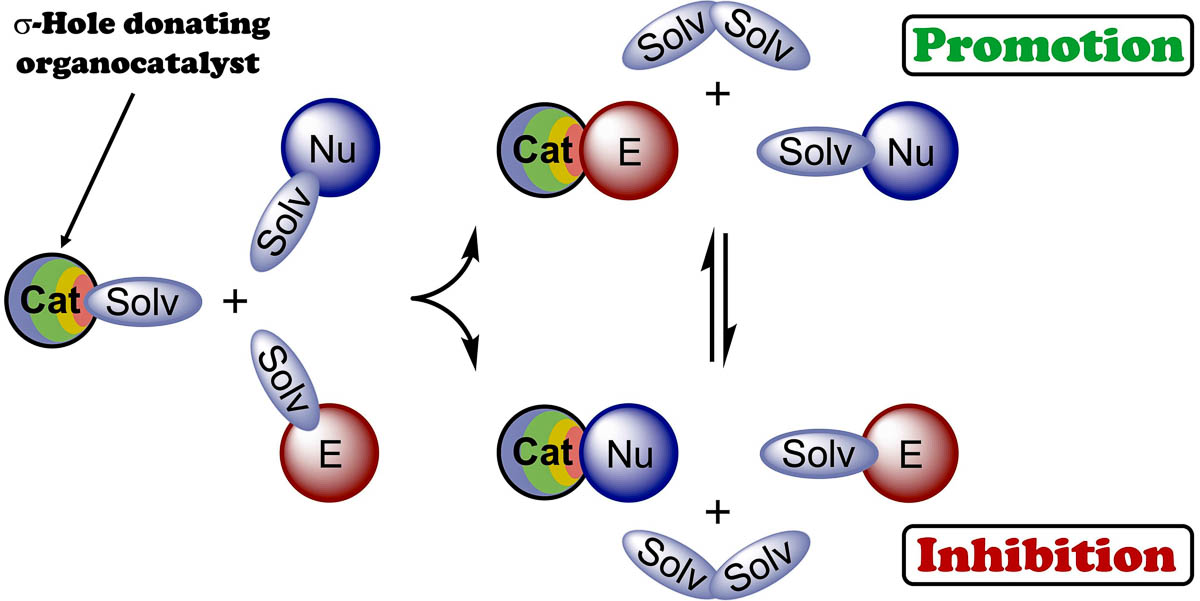 The combination of experimental data and results of DFT calculations indicates that the catalytic activity of chalconium and halonium salts serving as sigma-hole donating organocatalysts cannot be clearly estimated via analysis of the electrostatic potential on the catalysts’ sigma-holes and values of the catalyst•••TS intermolecular interactions, such as polarization effects, charge transfer, or covalency of bonding. Moreover, the real catalytic effect might not correlate well with the values of Gibbs free energy of activation of the reactions, because solvation effects and other competitive binding processes play at least an equal or even more important role in the catalysis. It was shown in the present work that the solvation can either lead to the increase of equilibrium concentration of reactive catalyst•••electrophile associates, thus accelerating the reaction, or brings favorable generation of catalyst•••nucleophile species resulting in the suppression of the catalytic activity of the organocatalyst.
The combination of experimental data and results of DFT calculations indicates that the catalytic activity of chalconium and halonium salts serving as sigma-hole donating organocatalysts cannot be clearly estimated via analysis of the electrostatic potential on the catalysts’ sigma-holes and values of the catalyst•••TS intermolecular interactions, such as polarization effects, charge transfer, or covalency of bonding. Moreover, the real catalytic effect might not correlate well with the values of Gibbs free energy of activation of the reactions, because solvation effects and other competitive binding processes play at least an equal or even more important role in the catalysis. It was shown in the present work that the solvation can either lead to the increase of equilibrium concentration of reactive catalyst•••electrophile associates, thus accelerating the reaction, or brings favorable generation of catalyst•••nucleophile species resulting in the suppression of the catalytic activity of the organocatalyst.
2022
Novikov A.S., Bolotin D.S., Хеnon Derivatives as Aerogen Bond-Donating Catalysts for Organic Transformations: A Theoretical Study on the Metaphorical "Spherical Cow in a Vacuum" Provides Insights into Noncovalent Organocatalysis, J. Org. Chem., 2023, 88, 1936–1944. DOI: 10.1021/acs.joc.2c00680
Computations indicate that cationic and non-charged xenon derivatives should exhibit higher catalytic activity than their iodine-based noncovalent organocatalytic congeners. Perfluorophenyl xenonium(II) is expected to demonstrate the best balance between catalytic activity and chemical stability for use in organocatalysis. Comparing its catalytic activity with that of isoelectronic perfluoroiodobenzene indicates that the high catalytic activity of cationic noncovalent organocatalysts is predominantly attributed to the electrostatic interactions with the reaction substrates, which cause the polarization of the ligated species during the reaction progress. In contrast, the electron transfer and covalent contributions to the bonding between the catalyst and substrate have negligible effects. The dominant effect of the electrostatic interactions results in a strong negative correlation between the calculated Gibbs free energies of activation for the modeled reactions and the highest potentials of the σ-holes on the central atoms of the catalysts. No such correlation is observed for the non-charged catalysts.
Polonnikov D.A., Il'in M.V., Safinskay Ya.V., Aliyarova I.S., Novikov A.S., Bolotin D.S., (Pre)association as a Crucial Step for Computational Prediction and Analysis of the Catalytic Activity of Sigma-Hole Donating Organocatalysts, Org. Chem. Front., 2023, 10, 169–180. DOI: 10.1039/D2QO01648F
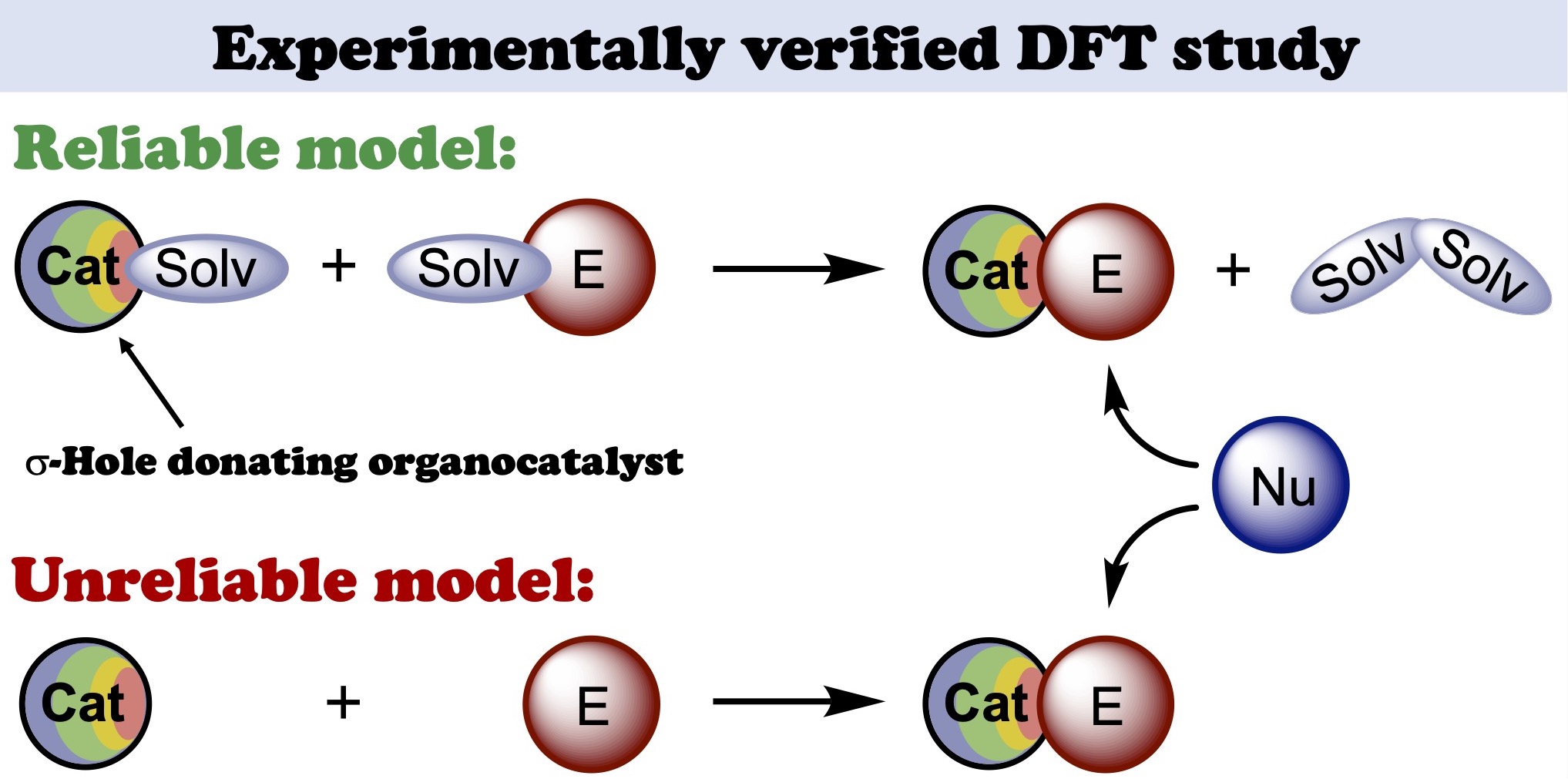 Based upon the experimentally obtained kinetic data on iodonium salt catalyzed nucleophilic addition of isocyanide to imine leading to imidazopyridine species, a reliable model for DFT calculations has been suggested. It was shown that preassociation of the catalysts with the reaction species might significantly affect the total energy profile of the reaction obtained by DFT. The associates of the organocatalysts featuring the solvent molecules ligated to its sigma-holes should be used as a starting point for calculations. Taking these reaction steps into consideration during the calculations leads to significantly more reliable theoretical results, which can be applied either for prediction of the catalytic activity of yet untested species or for justification of the catalytic paths based upon the experimental kinetic data as well.
Based upon the experimentally obtained kinetic data on iodonium salt catalyzed nucleophilic addition of isocyanide to imine leading to imidazopyridine species, a reliable model for DFT calculations has been suggested. It was shown that preassociation of the catalysts with the reaction species might significantly affect the total energy profile of the reaction obtained by DFT. The associates of the organocatalysts featuring the solvent molecules ligated to its sigma-holes should be used as a starting point for calculations. Taking these reaction steps into consideration during the calculations leads to significantly more reliable theoretical results, which can be applied either for prediction of the catalytic activity of yet untested species or for justification of the catalytic paths based upon the experimental kinetic data as well.
Novikov A.S., Bolotin D.S., Halonium, Chalconium, and Pnictonium Salts as Noncovalent Organocatalysts: A Computational Study on Relative Catalytic Activity, Org. Biomol. Chem., 2022, 20, 7632–7639. DOI: 10.1039/D2OB01415G
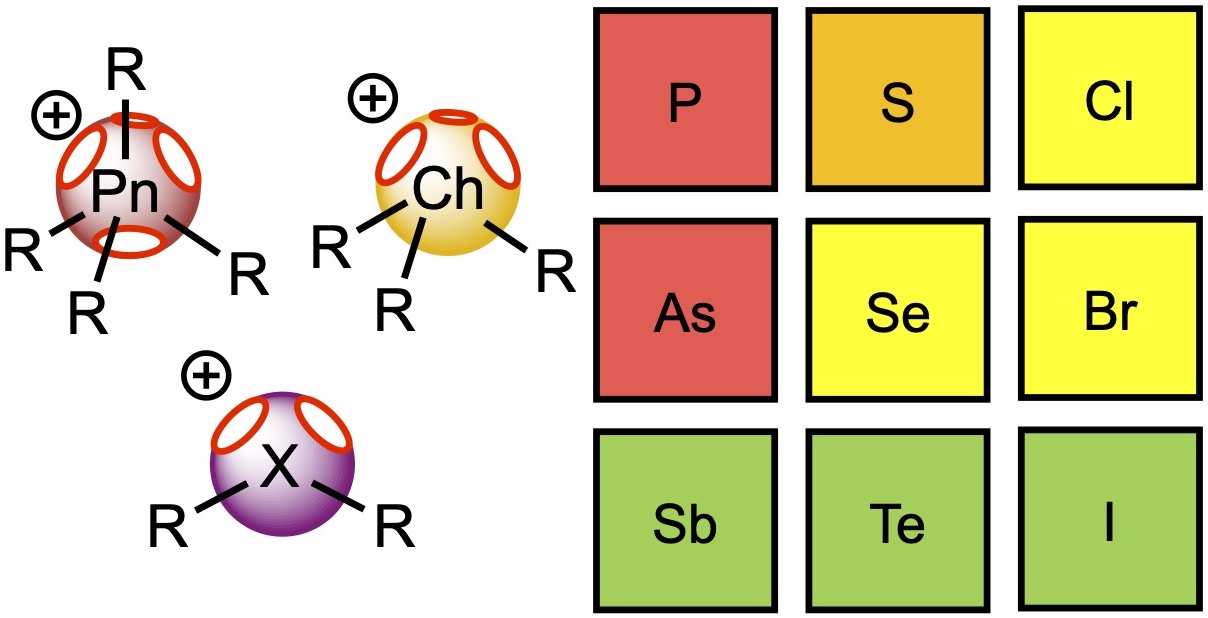 This theoretical study sheds light on the relative catalytic activity of pnictonium, chalconium, and halonium salts in reactions involving elimination of chloride and electrophilic activation of a carbonyl group. DFT calculations indicate that for cationic aromatic onium salts, values of the electrostatic potential on heteroatom σ-holes gradually increase from pnictogen- to halogen-containing species. The higher values of the potential on the halogen atoms of halonium salts result in the overall higher catalytic activity of these species, but in the case of pnictonium and chalconium cations, weak interactions from the side groups provide an additional stabilization effect on the reaction transition states. Based upon quantum-chemical calculations, the catalytic activity of phosphonium(V) and arsenonium(V) salts is expected to be too low to obtain effective noncovalent organocatalytic compounds, whereas stibonium(V), telluronium(IV) and iodonium(III) salts exhibit higher potential in application as noncovalent organocatalysts.
This theoretical study sheds light on the relative catalytic activity of pnictonium, chalconium, and halonium salts in reactions involving elimination of chloride and electrophilic activation of a carbonyl group. DFT calculations indicate that for cationic aromatic onium salts, values of the electrostatic potential on heteroatom σ-holes gradually increase from pnictogen- to halogen-containing species. The higher values of the potential on the halogen atoms of halonium salts result in the overall higher catalytic activity of these species, but in the case of pnictonium and chalconium cations, weak interactions from the side groups provide an additional stabilization effect on the reaction transition states. Based upon quantum-chemical calculations, the catalytic activity of phosphonium(V) and arsenonium(V) salts is expected to be too low to obtain effective noncovalent organocatalytic compounds, whereas stibonium(V), telluronium(IV) and iodonium(III) salts exhibit higher potential in application as noncovalent organocatalysts.
Il'in M.V., Novikov A.S., Bolotin D.S., Sulfonium and Selenonium Salts as Noncovalent Organocatalysts for the Multicomponent Groebke–Blackburn–Bienaymé Reaction, J. Org. Chem., 2022, 87, 10199–10207. DOI: 10.1021/acs.joc.2c01141
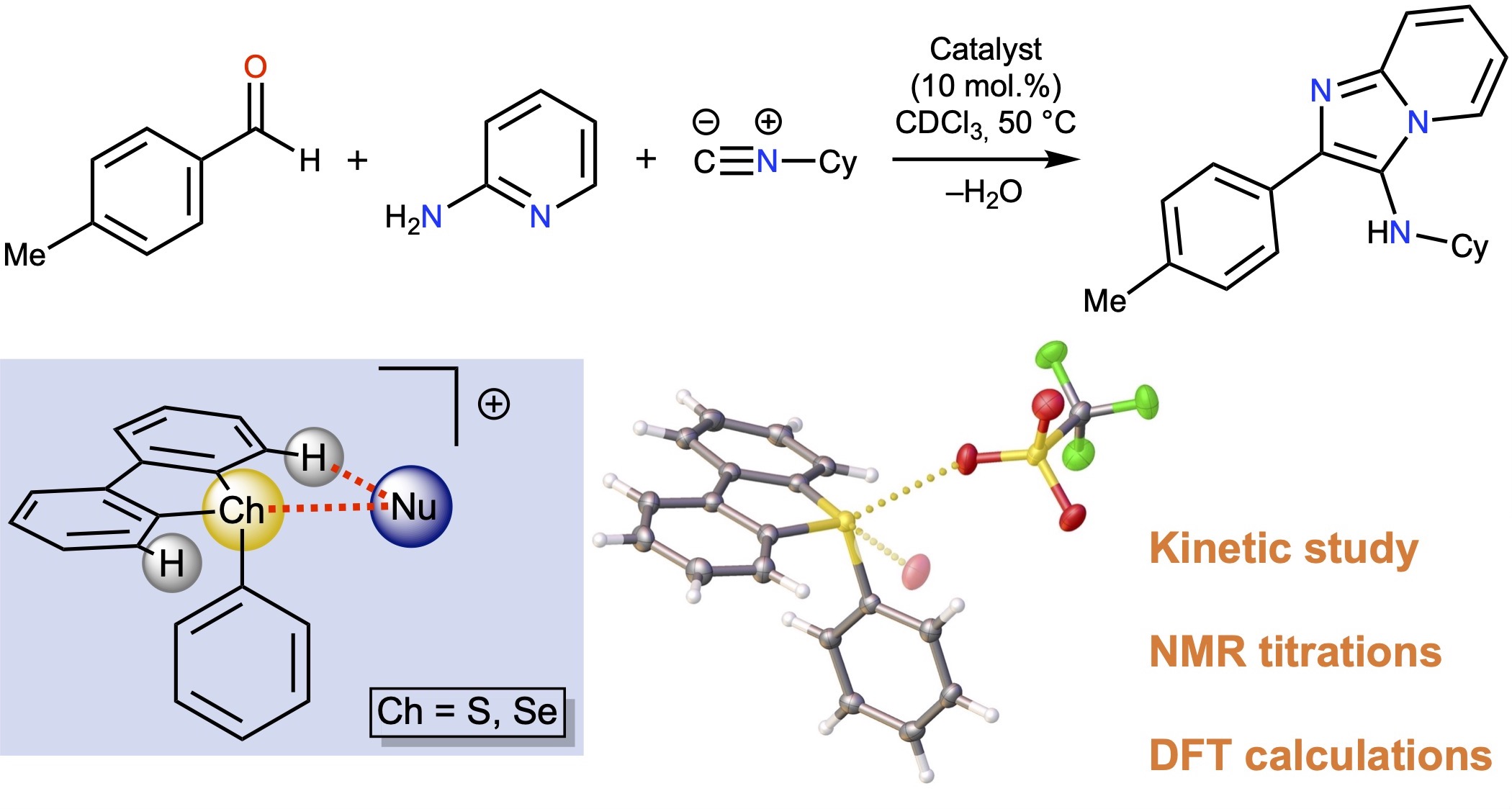 Sulfonium and selenonium salts, represented by S-aryl dibenzothiophenium and Se-aryl dibenzoselenophenium triflates, were found to exhibit remarkable catalytic activity in the model Groebke–Blackburn–Bienaymé reaction. Kinetic analysis and density functional theory (DFT) calculations indicated that their catalytic effect is induced by the ligation of the reaction substrates to the σ-holes on the S or Se atom of the cations. The experimental data indicated that although 10-fold excess of the chloride totally inhibits the catalytic activity of the sulfonium salts, the selenonium salt remains catalytically active, which can be explained by the experimentally found lower binding constant of the selenonium derivative to chloride in comparison with the sulfonium analogue. Both types of salts exhibit lower catalytic activity in the model reaction than dibenziodolium species.
Sulfonium and selenonium salts, represented by S-aryl dibenzothiophenium and Se-aryl dibenzoselenophenium triflates, were found to exhibit remarkable catalytic activity in the model Groebke–Blackburn–Bienaymé reaction. Kinetic analysis and density functional theory (DFT) calculations indicated that their catalytic effect is induced by the ligation of the reaction substrates to the σ-holes on the S or Se atom of the cations. The experimental data indicated that although 10-fold excess of the chloride totally inhibits the catalytic activity of the sulfonium salts, the selenonium salt remains catalytically active, which can be explained by the experimentally found lower binding constant of the selenonium derivative to chloride in comparison with the sulfonium analogue. Both types of salts exhibit lower catalytic activity in the model reaction than dibenziodolium species.
Il'in M.V., Sysoeva A.A., Novikov A.S., Bolotin D.S., Diaryliodoniums as Hybrid Hydrogen- and Halogen-Bond-Donating Organocatalysts for the Groebke–Blackburn–Bienaymé Reaction. J. Org. Chem., 2022, 87, 4569–4579. DOI: 10.1021/acs.joc.1c02885
 Dibenziodolium and diphenyliodonium triflates display high catalytic activity for the multicomponent reaction that leads to a series of imidazopyridines. Density functional theory (DFT) calculations indicate that both the salts can play the role of hybrid hydrogen- and halogen-bond-donating organocatalysts, which electrophilically activate the carbonyl and imine groups during the reaction process. The ortho-H atoms in the vicinal position to the I atom play a dual role: forming additional noncovalent bonds with the ligated substrate and increasing the maximum electrostatic potential on the σ-hole at the iodine atom owing to the effects of polarization. Dibenziodolium triflate exhibits higher catalytic activity, and the results obtained from 1H nuclear magnetic resonance (NMR) titrations, in conjunction with those from DFT calculations, indicate that this could be explained in terms of the additional energy required for the rotation of the phenyl ring in the diphenyliodonium cation during ligation of the substrate.
Dibenziodolium and diphenyliodonium triflates display high catalytic activity for the multicomponent reaction that leads to a series of imidazopyridines. Density functional theory (DFT) calculations indicate that both the salts can play the role of hybrid hydrogen- and halogen-bond-donating organocatalysts, which electrophilically activate the carbonyl and imine groups during the reaction process. The ortho-H atoms in the vicinal position to the I atom play a dual role: forming additional noncovalent bonds with the ligated substrate and increasing the maximum electrostatic potential on the σ-hole at the iodine atom owing to the effects of polarization. Dibenziodolium triflate exhibits higher catalytic activity, and the results obtained from 1H nuclear magnetic resonance (NMR) titrations, in conjunction with those from DFT calculations, indicate that this could be explained in terms of the additional energy required for the rotation of the phenyl ring in the diphenyliodonium cation during ligation of the substrate.


